^
Per your order, we have compounded ACD Solution Modified as a solution of 10 mL in a 100 mL vial. The characteristics of this compounded preparation are as follows
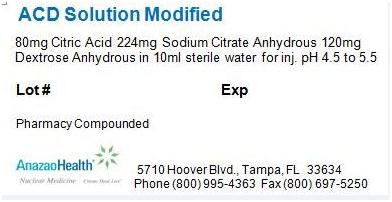
^Description
Each 100 mL vial contains 80 mg citric acid, 224 mg sodium citrate anhydrous, and 120 mg dextrose anhydrous in a sterile, non-pyrogenic solution of 10 mL. The pH of the solution has been adjusted to be between 4.5 to 5.5
^Dosage And Administration
Red Blood Cell Labeling Procedure
^Clinical Pharmacology
In vitro, citrate ions combine with ionic calcium in the blood and the resulting
lack of ionic calcium prevents coagulation. Blood that has been treated with citrate anticoagulants is nontoxic to the body when injected in small amounts intravenously. After injection, citrate ions are rapidly removed from the blood by the liver, polymerized into glucose, and then metabolized in the usual manner
^Package Label.principal Display Panel
Figure 1
^Indications And Usage
ACD solution modified is to be used in the labeling of red blood cells for intravenous administration with Cr-51 Sodium Chromate.
^Storage And Handling
Store the product at room temperature
^Contraindications
There are no known contraindications.