^Overdosage:
Topically applied corticosteroids can be absorbed in sufficient amounts to produce systemic effects (see PRECAUTIONS ).
^Adverse Reactions:
The following local adverse reactions are reported infrequently with topical corticosteroids, but may occur more frequently with the use of occlusive dressings. These reactions are listed in an approximate decreasing order of occurrence: burning, itching, irritation, dryness, folliculitis, hypertrichosis, acneiform eruptions, hypopigmentation, perioral dermatitis, allergic contact dermatitis, maceration of the skin, secondary infection, skin atrophy, striae and miliaria.
^Clinical Pharmacology:
Topical corticosteroids share anti-inflammatory, antipruritic and vasoconstrictive actions.
The mechanism of anti-inflammatory activity of the topical corticosteroids is unclear. Various laboratory methods, including vasoconstrictor assays, are used to compare and predict potencies and/or clinical efficacies of the topical corticosteroids. There is some evidence to suggest that a recognizable correlation exists between vasoconstrictor potency and therapeutic efficacy in man.
^Dosage And Administration:
Topical corticosteroids are generally applied to the affected area as a thin film from two to three times daily depending on the severity of the condition.
Occlusive dressings may be a valuable therapeutic adjunct for the management of psoriasis or recalcitrant conditions.
If an infection develops, the use of occlusive dressings should be discontinued and appropriate antimicrobial therapy instituted.
^Description:
The topical corticosteroids constitute a class of primarily synthetic steroids used as anti-inflammatory and antipruritic agents.
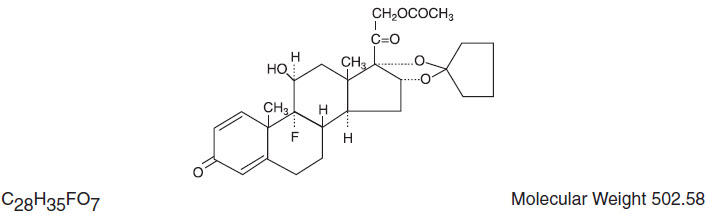
Each gram of Amcinonide Ointment USP, 0.1% contains 1 mg of the active steroid amcinonide in a specially formulated base composed of Benzyl Alcohol 2%, (wt/wt) as preservative, White Petrolatum, USP, Emulsifying Wax, and Antioxidant Blend (Propylene Glycol, Butylated Hydroxyanisole, Propyl Gallate and Citric Acid). Chemically, amcinonide is:
Pregna-1,4-diene-3,20-dione,21-(acetyloxy)-16,17-[cyclopentylidenebis(oxy)]-9-fluoro-11-hydroxy-, (11β, 16α).
^Package Label – Principal Display Panel – G Container
NDC 0168-0279-60
FOUGERA®
AMCINONIDEOINTMENT USP, 0.1%
Rx only NOT FOR OPHTHALMIC USE.FOR DERMATOLOGIC USE ONLY.
NET WT 60 grams

^Package Label – Principal Display Panel – G Carton
NDC 0168-0279-60
Rx only
FOUGERA®
AMCINONIDEOINTMENT USP, 0.1%
NOT FOR OPHTHALMIC USE.FOR DERMATOLOGIC USE ONLY.
NET WT 60 grams

^Indications And Usage:
Topical corticosteroids are indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid-responsive dermatoses.
^Contraindications:
Topical corticosteroids are contraindicated in those patients with a history of hypersensitivity to any of the components of the preparation.
^How Supplied:
Amcinonide Ointment USP, 0.1% (1 mg/g) is supplied as follows: NDC 0168-0279-60 60 gram tubes
Store at controlled room temperature 15° - 30°C (59° - 86°F) (see USP).DO NOT FREEZE.
E. FOUGERA & CO. A division of Fougera PHARMACEUTICALS INC.Melville, New York 11747
I2279DR08/12#78