^ Serious Fluid And Electrolyte Abnormalities
Advise patients to hydrate adequately before, during, and after the use of CLENPIQ. Use caution in patients with congestive heart failure when replacing fluids. If a patient develops significant vomiting or signs of dehydration including signs of orthostatic hypotension after taking CLENPIQ, consider performing post-colonoscopy lab tests (electrolytes, creatinine, and BUN) and treat accordingly. Approximately 20% of patients in both arms (sodium picosulfate, magnesium oxide, and anhydrous citric acid, or 2 L of PEG + E plus two × 5-mg bisacodyl tablets) of clinical trials of another oral sodium picosulfate, magnesium oxide, and anhydrous citric acid product had orthostatic changes in blood pressure and/or heart rate on the day of colonoscopy and up to seven days post colonoscopy. In a single study of patients 9 to 16 years of age, approximately 20% of patients who received another oral product of sodium picosulfate, magnesium oxide, and anhydrous citric acid had orthostatic changes (changes in blood pressure and/or heart rate) compared with approximately 7% of those who received the comparator (PEG) [see Clinical Studies (14)]. These changes occurred up to five days post colonoscopy.
Fluid and electrolyte disturbances can lead to serious adverse reactions including cardiac arrhythmias, seizures, renal impairment, and syncope [see Warnings and Precautions (5.5)]. Correct fluid and electrolyte abnormalities before treatment with CLENPIQ. Advise patients to consume a variety of clear liquids (e.g., balanced electrolyte solution), and not only water after each dose of CLENPIQ. In addition, use caution when prescribing CLENPIQ for patients who have conditions or who are using medications that increase the risk for fluid and electrolyte disturbances or that may increase the risk of seizure, arrhythmia, and renal impairment [see Drug Interactions (7.1)].
^ Pharmacodynamics
The stimulant laxative activity of sodium picosulfate together with the osmotic laxative activity of magnesium citrate produces a purgative effect which, when ingested with additional liquids, produces watery diarrhea.
^ Colonic Mucosal Ulceration, Ischemic Colitis, And Ulcerative Colitis
Osmotic laxatives may produce colonic mucosal aphthous ulcerations and there have been reports of more serious cases of ischemic colitis requiring hospitalization. Concurrent use of additional stimulant laxatives with CLENPIQ may increase this risk. Consider the potential for mucosal ulcerations when interpreting colonoscopy findings in patients with known or suspected inflammatory bowel disease [see Adverse Reactions (6.2)].
^ Potential For Reduced Drug Absorption
CLENPIQ can reduce the absorption of other co-administered drugs [see Dosage and Administration (2.1)]:
^ Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide and Instructions for Use).
Instruct patients:
^ Description
CLENPIQ (sodium picosulfate, magnesium oxide, and anhydrous citric acid) oral solution is a stimulant and osmotic laxative that is provided as a cranberry-flavored, colorless to slightly yellow, clear solution with possible presence of visible particles. CLENPIQ is supplied as two bottles in each carton.
Each bottle of CLENPIQ contains 10 mg sodium picosulfate, USP; 3.5 g magnesium oxide, USP; and 12 g anhydrous citric acid, USP. The product also contains the following inactive ingredients:
acesulfame potassium, cranberry flavor, disodium edetate, malic acid, sodium benzoate, sodium hydroxide, sodium metabisulfite, sucralose, and water. The cranberry flavor contains glyceryl triacetate (triacetin), maltodextrin, and sodium octenyl succinated starch.
The following is a description of the three active ingredients contained in CLENPIQ:
Sodium picosulfate is a stimulant laxative.
^ Antibiotics
Prior or concomitant use of antibiotics with CLENPIQ may reduce efficacy of CLENPIQ as conversion of sodium picosulfate to its active metabolite BHPM is mediated by colonic bacteria.
^ Overdosage
Overdosage of more than the recommended dose of CLENPIQ may lead to severe electrolyte disturbances, as well as dehydration and hypovolemia, with signs and symptoms of these disturbances [see Warnings and Precautions (5.1)]. Monitor for fluid and electrolyte disturbances and treat symptomatically.
^ Seizures
There have been reports of generalized tonic-clonic seizures and/or loss of consciousness with the use of bowel preparation products in patients with no prior history of seizures. The seizure cases were associated with electrolyte abnormalities (e.g., hyponatremia, hypokalemia, hypocalcemia, and hypomagnesemia) and low serum osmolality. The neurologic abnormalities resolved with correction of fluid and electrolyte abnormalities.
Use caution when prescribing CLENPIQ for patients with a history of seizures and in patients at risk of seizure, such as patients taking medications that lower the seizure threshold (e.g., tricyclic antidepressants), patients withdrawing from alcohol or benzodiazepines, patients with known or suspected hyponatremia [see Adverse Reactions (6.2)].
^ Renal Impairment
CLENPIQ is contraindicated in patients with severe renal impairment (creatinine clearance less than 30 mL/min), as accumulation of magnesium in plasma may occur [see Contraindications (4)]. Patients with less severe renal impairment or patients taking concomitant medications that may affect renal function may be at increased risk for renal injury [see Warnings and Precautions (5.3)]. Advise these patients of the importance of adequate hydration before, during, and after the use of CLENPIQ [see Dosage and Administration (2.1)]. Consider performing baseline and post-colonoscopy laboratory tests (electrolytes, creatinine, and BUN) in these patients.
^3 Dosage Forms And Strengths
Oral solution: Each bottle contains 10 mg of sodium picosulfate, 3.5 grams of magnesium oxide, and 12 grams of anhydrous citric acid in 175 mL of colorless to slightly yellow, clear solution with possible presence of visible particles.
^ Important Administration Instructions
^ Pediatric Use
The safety and effectiveness of CLENPIQ have been established for cleansing of the colon as a preparation for colonoscopy in pediatric patients 9 years of age and older. Use of CLENPIQ in this age group is supported by evidence from adequate and well-controlled trials in adults and a single, dose-ranging, controlled trial in 78 pediatric patients 9 to 16 years of age all of which evaluated another oral product of sodium picosulfate, magnesium oxide, and anhydrous citric acid [see Clinical Studies (14)]. The safety profile in this pediatric population was similar to that seen in adults [see Adverse Reactions (6.1)]. Monitor for possible hypoglycemia in pediatric patients, as CLENPIQ has no caloric substrate.
The safety and effectiveness of CLENPIQ in pediatric patients less than 9 years of age have not been established.
^4 Contraindications
CLENPIQ is contraindicated in the following conditions:
^ Use In Patients With Renal Impairment
CLENPIQ is contraindicated in patients with severe renal impairment (creatinine clearance less than 30 mL/min), accumulation of magnesium in plasma may occur. Use caution when prescribing CLENPIQ for patients with mild to moderate renal impairment or patients taking concomitant medications that may affect renal function (such as diuretics, angiotensin-converting enzyme inhibitors, angiotensin receptor blockers, or non-steroidal anti-inflammatory drugs) [see Drug Interactions (7.1)]. These patients may be at increased risk for renal injury. Advise these patients of the importance of adequate hydration before, during, and after the use of CLENPIQ. Consider performing baseline and post-colonoscopy laboratory tests (electrolytes, creatinine, and BUN) in these patients.
^ Geriatric Use
Of the 448 adult patients in Study 1 who received CLENPIQ, 124 (28%) patients were 65 years of age or older. No overall differences in safety or effectiveness were observed between geriatric patients and younger patients, and other reported clinical experience has not identified differences in responses between elderly and younger patients. Elderly patients are more likely to have decreased hepatic, renal, or cardiac function and may be more susceptible to adverse reactions resulting from fluid and electrolyte abnormalities [see Warnings and Precautions (5.1)].
^ Carcinogenesis, Mutagenesis, Impairment Of Fertility
Long-term studies in animals to evaluate carcinogenic potential or studies to evaluate mutagenic potential have not been performed with CLENPIQ.
Sodium picosulfate was not mutagenic in the Ames test, the mouse lymphoma assay, and the mouse bone marrow micronucleus test.
In an oral fertility study in rats, sodium picosulfate, magnesium oxide, and anhydrous citric acid did not cause any significant adverse effect on male or female fertility parameters up to a maximum dose of 2000 mg/kg twice daily (about 1.2 times the recommended human dose based on body surface area).
^Instructions For Useclenpiq® (clen-pik)(sodium Picosulfate, Magnesium Oxide, And Anhydrous Citric Acid) Oral Solution
READ BEFORE Taking CLENPIQ
If you have questions before you start CLENPIQ, talk to your healthcare provider.
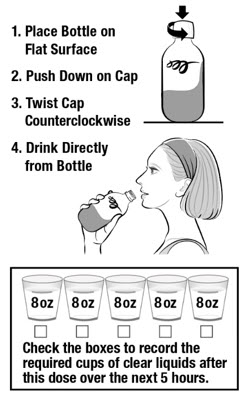
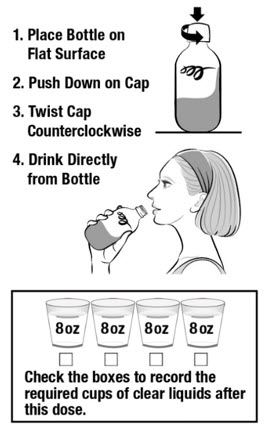
Take CLENPIQ using the Split-Dose method. This means you will take 2 separate doses. Each bottle is 1 dose and has the same ingredients. You can drink either bottle first.
Start a clear-liquid diet the day before your colonoscopy. Do not eat any solid foods. Drink a variety of clear liquids. Clear liquids should include balanced electrolyte solution such as sports drinks (see Table 1).
You must drink enough clear liquids to keep hydrated.
IMPORTANT: You can drink the clear liquids in Table 1.
IMPORTANT: Do NOT eat or drink the items in Table 2 starting from the day before your colonoscopy.
SPLIT-DOSE INSTRUCTIONS
DOSE 1
In the evening the day before your colonoscopy (sometime between 5:00 PM to 9:00 PM)
IMPORTANT: See Table 1 for a list of clear liquids you can drink.
If you have any bloating or upset stomach after drinking CLENPIQ, wait until your stomach feels better before taking your second dose.
DOSE 2
In the morning of your colonoscopy (about 5 hours before your colonoscopy)
Do not eat solid food. Drink only clear liquids. Do not drink only plain water (see Table 1).
IMPORTANT: See Table 1 for a list of clear liquids you can drink.
This Instructions for Use has been approved by the U.S. Food and Drug Administration.
Ferring Pharmaceuticals Inc. Parsippany, NJ 07054, USARevised 08/20232009010037
^ Split-dose Dosage Regimen
The recommended dosage in adults and pediatric patients 9 years of age and older is shown below. Instruct patients to take two separate doses in conjunction with liquids, as follows:
^Principal Display Panel - Ml Bottle Carton
CranberryFlavorNDC 55566-6800-1
CLENPIQ®
(sodium picosulfate, magnesium oxide,and anhydrous citric acid) Oral Solution
10 mg/3.5 g/12 g per 175 mL bottle
CLENPIQ® is a ready-to-drinkoral solution that doesn't need to be diluted.
Read the enclosed Instructions for Use and Medication GuideAT LEAST 2 DAYS BEFORE your colonoscopyand again right before taking CLENPIQ®.
Contains two (2) 175 mL bottles
Do not refrigerate or freeze.
Rx only
FERRINGPHARMACEUTICALS

^ Syncope
Syncope has been reported with CLENPIQ in the postmarketing setting. Some cases were serious events that included falls with associated head injuries or fractures requiring hospitalization. In some cases, electrolyte abnormalities were also present (e.g., hyponatremia and hypokalemia). Cases have been reported after one or two CLENPIQ doses and many of these cases occurred within 12 hours of dosing. Patients should be aware of the risk of syncope during treatment and adequately hydrate before, during, and after the use of CLENPIQ. Advise patients to consume a variety of clear liquids (e.g., balanced electrolyte solution), not only water after each dose of CLENPIQ and to get up gradually from a lying or sitting position [see Warnings and Precautions (5.1)].
^6 Adverse Reactions
The following serious or otherwise important adverse reactions for bowel preparations are described elsewhere in the labeling:
^ Drugs That May Increase Risks Of Fluid And Electrolyte Abnormalities
Use caution when prescribing CLENPIQ for patients with conditions or who are taking other drugs, that increase the risk for fluid and electrolyte disturbances or may increase the risk of renal impairment, seizures, syncope, arrhythmias or QT prolongation in the setting of fluid and electrolyte abnormalities [see Warnings and Precautions (5.1, 5.2, 5.3, 5.4, 5.5)].
^ Postmarketing Experience
The following adverse reactions have been identified during post approval use of oral sodium picosulfate, magnesium oxide, and anhydrous citric acid products. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Hypersensitivity: rash, urticaria, and purpura
Gastrointestinal: abdominal pain, diarrhea, fecal incontinence, proctalgia, vomiting, reversible aphthoid ileal ulcers, and ischemic colitis [see Warnings and Precautions (5.5)]
Neurologic: generalized tonic-clonic seizures with and without hyponatremia in epileptic patients, and syncope [see Warnings and Precautions (5.2, 5.5)].
^ Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in practice.
^1 Indications And Usage
CLENPIQ is indicated for cleansing of the colon as a preparation for colonoscopy in adults and pediatric patients 9 years of age and older.
^ Use In Patients With Significant Gastrointestinal Disease
If gastrointestinal obstruction or perforation is suspected, perform appropriate diagnostic studies to rule out these conditions before administering CLENPIQ [see Contraindications (4)]. Use with caution in patients with severe active ulcerative colitis.
^ Mechanism Of Action
Sodium picosulfate is hydrolyzed by colonic bacteria to form an active metabolite: bis-(p-hydroxy-phenyl)-pyridyl-2-methane, BHPM, which acts directly on the colonic mucosa to stimulate colonic peristalsis. Magnesium oxide and citric acid react to create magnesium citrate in solution, which is an osmotic agent that causes water to be retained within the gastrointestinal tract.
^ Cardiac Arrhythmias
There have been rare reports of serious arrhythmias associated with the use of ionic osmotic laxative products for bowel preparation. Use caution when prescribing CLENPIQ for patients at increased risk of arrhythmias (e.g., patients with a history of prolonged QT, uncontrolled arrhythmias, recent myocardial infarction, unstable angina, congestive heart failure, or cardiomyopathy). Consider pre-dose and post-colonoscopy ECGs in patients at increased risk of serious cardiac arrhythmias.
^ Aspiration
Patients with impaired gag reflex are at risk for regurgitation or aspiration during the administration of CLENPIQ. Observe these patients during the administration of CLENPIQ.