^
^ Pharmacodynamics
In vitroand in vivostudies in animals suggest that escitalopram is a highly selective serotonin reuptake inhibitor (SSRI) with minimal effects on norepinephrine and dopamine neuronal reuptake. Escitalopram is at least 100-fold more potent than the R-enantiomer with respect to inhibition of 5-HT reuptake and inhibition of 5-HT neuronal firing rate. Tolerance to a model of antidepressant effect in rats was not induced by long-term (up to 5 weeks) treatment with escitalopram. Escitalopram has no or very low affinity for serotonergic (5-HT 1-7) or other receptors including alpha- and beta-adrenergic, dopamine (D 1 to 5), histamine (H 1 to t3), muscarinic (M 1 to 5), and benzodiazepine receptors. Escitalopram also does not bind to, or has low affinity for, various ion channels including Na +, K +, Cl -, and Ca ++channels. Antagonism of muscarinic, histaminergic, and adrenergic receptors has been hypothesized to be associated with various anticholinergic, sedative, and cardiovascular side effects of other psychotropic drugs.
^ Discontinuation Syndrome
During marketing of escitalopram oxalate and other SSRIs, there have been spontaneous reports of adverse reactions occurring upon discontinuation of these drugs, particularly when abrupt, including the following: dysphoric mood, irritability, agitation, dizziness, sensory disturbances (e.g., paresthesias such as electric shock sensations), anxiety, confusion, headache, lethargy, emotional lability, insomnia, and hypomania. While these events are generally self-limiting, there have been reports of serious discontinuation symptoms.
Monitor for these symptoms when discontinuing treatment with escitalopram oxalate. A gradual reduction in the dose rather than abrupt cessation is recommended whenever possible. If intolerable symptoms occur following a decrease in the dose or upon discontinuation of treatment, then resuming the previously prescribed dose may be considered. Subsequently, the physician may continue decreasing the dose but at a more gradual rate [ see Dosage and Administration ( 2.6) ].
^ Increased Risk Of Bleeding
Drugs that interfere with serotonin reuptake inhibition, including escitalopram oxalate, increase the risk of bleeding events. Concomitant use of aspirin, nonsteroidal anti-inflammatory drugs (NSAIDs), other antiplatelet drugs, warfarin, and other anticoagulants may add to the risk. Case reports and epidemiological studies (case-control and cohort design) have demonstrated an association between use of drugs that interfere with serotonin reuptake and the occurrence of gastrointestinal bleeding. Based on data from the published observational studies, exposure to SSRIs, particularly in the month before delivery, has been associated with a less than 2-fold increase in the risk of postpartum hemorrhage [see Use in Specific Populations (8.1)]. Bleeding events related to drugs that interfere with serotonin reuptake have ranged from ecchymoses, hematomas, epistaxis, and petechiae to life-threatening hemorrhages.
Inform patients about the increased risk of bleeding associated with the concomitant use of escitalopram oxalate and antiplatelet agents or anticoagulants. For patients taking warfarin, carefully monitor the international normalized ratio [see Drug Interactions ( 7 )].
^ Description
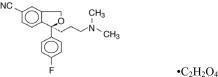
Escitalopram oxalate contains escitalopram, a selective serotonin reuptake inhibitor (SSRI), present as escitalopram oxalate salt. Escitalopram is the pure S-enantiomer (single isomer) of the racemic bicyclic phthalane derivative citalopram. Escitalopram oxalate is designated S-(+)-1-[3(dimethyl-amino)propyl]-1-( p-fluorophenyl)-5-phthalancarbonitrile oxalate with the following structural formula:
The molecular formula is C 20H 21FN 2O• C 2H 2O 4and the molecular weight is 414.40.
Escitalopram oxalate occurs as a fine, white to slightly-yellow powder and is freely soluble in methanol and dimethyl sulfoxide (DMSO), soluble in isotonic saline solution, sparingly soluble in water and ethanol, slightly soluble in ethyl acetate, and insoluble in heptane.
Escitalopram tablets, USP are film-coated, round tablets containing 6.38 mg, 12.75 mg, 25.50 mg escitalopram oxalate in strengths equivalent to 5 mg, 10 mg, and 20 mg, respectively of escitalopram base. The 10 and 20 mg tablets are scored. The tablets also contain the following inactive ingredients: croscarmellose sodium, microcrystalline cellulose, hypromellose, colloidal anhydrous silica, magnesium stearate and talc. The film coating contains hypromellose, titanium dioxide, and polyethylene glycol.
^ Switching Patients To Or From A Monoamine Oxidase Inhibitor (maoi) Antidepressant
At least 14 days should elapse between discontinuation of an MAOI intended to treat psychiatric disorders and initiation of therapy with escitalopram tablets. Conversely, at least 14 days should be allowed after stopping escitalopram tablets before starting an MAOI intended to treat psychiatric disorders [ see Contraindications ( 4) ].
^ Use In Patients With Concomitant Illness
Clinical experience with escitalopram oxalate in patients with certain concomitant systemic illnesses is limited. Caution is advisable in using escitalopram oxalate in patients with diseases or conditions that produce altered metabolism or hemodynamic responses.
Escitalopram oxalate has not been systematically evaluated in patients with a recent history of myocardial infarction or unstable heart disease. Patients with these diagnoses were generally excluded from clinical studies during the product's premarketing testing.
In subjects with hepatic impairment, clearance of racemic citalopram was decreased and plasma concentrations were increased. The recommended dose of escitalopram oxalate in hepatically impaired patients is 10 mg daily [ see Dosage and Administration ( 2.5)and Use in Specific Populations (8.6) ].
Because escitalopram is extensively metabolized, excretion of unchanged drug in urine is a minor route of elimination. Until adequate numbers of patients with severe renal impairment have been evaluated during chronic treatment with escitalopram oxalate, however, it should be used with caution in such patients [ see Dosage and Administration ( 2.5) and Use in Specific Populations ( 8.7) ].
^ Clinical Trials Experience
Because clinical studies are conducted under widely varying conditions, adverse reaction rates observed in the clinical studies of a drug cannot be directly compared to rates in the clinical studies of another drug and may not reflect the rates observed in practice.
^ Overdosage
The following have been reported with escitalopram oxalate tablet overdosage:
Prolonged cardiac monitoring is recommended in escitalopram oxalate overdosage ingestions due to the arrhythmia risk.
Gastrointestinal decontamination with activated charcoal should be considered in patients who present early after a escitalopram oxalate overdose.
Consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdose management recommendations.
^ Lactation
Risk Summary
Data from the published literature report the presence of escitalopram and desmethylescitalopram in human milk (see Data). There are reports of excessive sedation, restlessness, agitation, poor feeding and poor weight gain in infants exposed to escitalopram, through breast milk (see Clinical Considerations). There are no data on the effects of escitalopram or its metabolites on milk production. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for escitalopram oxalate and any potential adverse effects on the breastfed child from escitalopram oxalate or from the underlying maternal condition.
Clinical Considerations
Infants exposed to escitalopram oxalate should be monitored for excess sedation, restlessness, agitation, poor feeding and poor weight gain.
Data
A study of 8 nursing mothers on escitalopram with daily doses of 10 to 20 mg/day showed that exclusively breast-fed infants receive approximately 3.9% of the maternal weight-adjusted dose of escitalopram and 1.7% of the maternal weight-adjusted dose of desmethylcitalopram.
^ Hepatic Impairment
Increased citalopram exposure occurs in patients with hepatic impairment [see Clinical Pharmacology ( 12.3)]. The recommended dosage of escitalopram oxalate in patients with hepatic impairment is 10 mg daily [see Dosage and Administration ( 2.5)].
^Warning: Suicidal Thoughts And Behaviors
Antidepressants increased the risk of suicidal thoughts and behaviors in pediatric and young adult patients in short-term studies. Closely monitor all antidepressant-treated patients for clinical worsening, and for emergence of suicidal thoughts and behaviors [See Warnings and Precautions ( 5.1)]. Escitalopram oxalate is not approved for use in pediatric patients less than 7 years of age [see Use in Specific Populations( 8.4)].
^ Pediatric Use
Major Depressive Disorder
The safety and effectiveness of escitalopram oxalate for the treatment of major depressive disorder have been established in pediatric patients 12 years of age and older. Use of escitalopram oxalate for this indication is supported by evidence from adequate and well-controlled studies in adults with additional evidence from an 8-week, flexible-dose, placebo-controlled study that compared escitalopram oxalate 10 mg to 20 mg once daily to placebo in pediatric patients 12 to 17 years of age with major depressive disorder [see Clinical Studies ( 14.1)]. The safety of escitalopram oxalate was similar to adult patients with MDD [see Adverse Reactions ( 6.1)].
The safety and effectiveness of escitalopram oxalate for the treatment of major depressive disorder have not been established in pediatric patients younger than 12 years of age. In a 24-week, open- label safety study in 118 pediatric patients aged 7 to 11 years who had major depressive disorder, the safety findings were consistent with the known safety and tolerability profile for escitalopram oxalate.
Generalized Anxiety Disorder The safety and effectiveness of escitalopram oxalate for the treatment of generalized anxiety disorder have not been established in pediatric patients younger than 7 years of age.
Antidepressants increase the risk of suicidal thoughts and behaviors in pediatric patients [see Warnings and Precautions ( 5.1)]. Decreased appetite and weight loss have been observed in association with the use of SSRIs. Consequently, regular monitoring of weight and growth should be performed in children and adolescents treated with an SSRI such as escitalopram oxalate.
Juvenile Animal Toxicity Data In a juvenile animal study, male and female rats were administered escitalopram at 5, 40, or 80 mg/kg/day by oral gavage from postnatal day (PND) 21 to PND 69. A delay in sexual maturation was observed in both males and females at ≥ 40 mg/kg/day with a No Observed Adverse Effect Level (NOAEL) of 5 mg/kg/day. This NOAEL was associated with plasma AUC levels less than those measured at the maximum recommended dose (MRHD) in pediatrics (20 mg). However, there was no effect on reproductive function. Increased motor activity (both ambulatory and fine movements) was observed in females prior to daily dosing at ≥ 40 mg/kg/day (3.5 times the MRHD based on AUC levels). A reversible disruption of learning and memory function was observed in males at 80 mg/kg/day with a NOAEL of 40 mg/kg/day, which was associated with an AUC level 3.5 times those measured at the MRHD in pediatrics. There was no effect on learning and memory function in treated female rats.
Additional pediatric use information is approved for AbbVie Inc.’s LEXAPRO (escitalopram) tablets and LEXAPRO (escitalopram) oral solution. However, due to AbbVie Inc.’s marketing exclusivity rights, this drug product is not labeled with that information.
^ Screen For Bipolar Disorder Prior To Starting Escitalopram Tablets
Prior to initiating treatment with escitalopram tablets or another antidepressant, screen patients for a personal family history of bipolar disorder, mania, or hypomania [see Warnings and Precautions ( 5.5)].
^ Interference With Cognitive And Motor Performance
In a study in normal volunteers, escitalopram oxalate 10 mg daily did not produce impairment of intellectual function or psychomotor performance. Because any psychoactive drug may impair judgment, thinking, or motor skills, however, patients should be cautioned about operating hazardous machinery, including automobiles, until they are reasonably certain that escitalopram oxalate therapy does not affect their ability to engage in such activities.
^ Geriatric Use
Approximately 69 patients (6%) of the 1,144 patients receiving escitalopram in controlled trials of escitalopram oxalate in major depressive disorder and GAD were 60 years of age or older [see Clinical Studies ( 14.1, 14.2)]. The number of elderly patients in these trials was insufficient to adequately assess for possible differential efficacy and safety measures on the basis of age. Nevertheless, greater sensitivity of some elderly individuals to effects of escitalopram oxalate cannot be ruled out.
In two pharmacokinetic studies, escitalopram half-life was increased by approximately 50% in subjects 65 years and older as compared to young subjects and Cmax was unchanged [see Clinical Pharmacology ( 12.3)]. The recommended dosage of escitalopram oxalate for elderly patients is 10 mg daily [see Dosage and Administration ( 2.5)].
SSRIs, including escitalopram oxalate, have been associated with cases of clinically significant hyponatremia in elderly patients, who may be at greater risk for this adverse reaction [see Warnings and Precautions ( 5.6)].
Of 4,422 patients in clinical studies of racemic citalopram, 1,357 were 60 and over, 1,034 were 65 and over, and 457 were 75 and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, and other reported clinical experience has not identified differences in responses between the geriatric and younger patients, but again, greater sensitivity of some elderly individuals cannot be ruled out.
^ Renal Impairment
Pharmacokinetics of escitalopram oxalate in patients with a creatinine clearance less than 20 mL/minute has not been evaluated. No dosage adjustment is necessary for patients with mild or moderate renal impairment [see Dosage and Administration ( 2.5), Clinical Pharmacology ( 12.3)].
^ Serotonin Syndrome
SSRIs, including escitalopram oxalate, can precipitate serotonin syndrome, a potentially life-threatening condition. The risk is increased with concomitant use of other serotonergic drugs (including triptans, tricyclic antidepressants, fentanyl, meperidine, methadone, lithium, tramadol, tryptophan, buspirone, amphetamines, and St. John’s Wort) and with drugs that impair metabolism of serotonin, i.e., MAOIs [see Contraindications ( 4) and Drug Interactions ( 7)].
Serotonin syndrome symptoms may include mental status changes (e.g., agitation, hallucinations, delirium, and coma), autonomic instability (e.g., tachycardia, labile blood pressure, dizziness, diaphoresis, flushing, hyperthermia), neuromuscular symptoms (e.g., tremor, rigidity, myoclonus, hyperreflexia, incoordination) seizures, and/or gastrointestinal symptoms (e.g., nausea, vomiting, diarrhea).
The concomitant use of escitalopram oxalate with MAOIs is contraindicated. In addition, do not initiate escitalopram oxalate in a patient who is being treated with MAOIs such as linezolid or intravenous methylene blue. No reports involved the administration of methylene blue by other routes (such as oral tablets or local tissue injection). If it is necessary to initiate treatment with an MAOI such as linezolid or intravenous methylene blue in a patient taking escitalopram oxalate, discontinue escitalopram oxalate before initiating treatment with the MAOI [ see Contraindications ( 4) and Dosage and Administration ( 2.7) ].
Monitor all patients taking escitalopram oxalate for the emergence of serotonin syndrome. Discontinue treatment with escitalopram oxalate and any concomitant serotonergic agents immediately if the above symptoms occur, and initiate supportive symptomatic treatment. If concomitant use of escitalopram oxalate with other serotonergic drugs is clinically warranted, inform patients of the increased risk for serotonin syndrome and monitor for symptoms.
^ Activation Of Mania Or Hypomania
In patients with bipolar disorder, treating a depressive episode with escitalopram oxalate or another antidepressant may precipitate a mixed/manic episode. In placebo-controlled trials of escitalopram oxalate in major depressive disorder, activation of mania/hypomania was reported in one (0.1%) of 715 patients treated with escitalopram oxalate and in none of the 592 patients treated with placebo. One additional case of hypomania has been reported in association with escitalopram oxalate treatment. Activation of mania/hypomania has also been reported in a small proportion of patients with major affective disorders treated with racemic citalopram and other marketed drugs effective in the treatment of major depressive disorder. Prior to initiating treatment with escitalopram oxalate, screen patients for any personal or family history of bipolar disorder, mania, or hypomania [see Dosage and Administration ( 2.4)].
^ Sexual Dysfunction
Use of SSRIs, including escitalopram oxalate, may cause symptoms of sexual dysfunction [ see Adverse Reactions ( 6.1) ].In male patients, SSRI use may result in ejaculatory delay or failure, decreased libido, and erectile dysfunction. In female patients, SSRI use may result in decreased libido and delayed or absent orgasm.
It is important for prescribers to inquire about sexual function prior to initiation of escitalopram oxalate and to inquire specifically about changes in sexual function during treatment, because sexual function may not be spontaneously reported. When evaluating changes in sexual function, obtaining a detailed history (including timing of symptom onset) is important because sexual symptoms may have other causes, including the underlying psychiatric disorder. Discuss potential management strategies to support patients in making informed decisions about treatment.
^ Hyponatremia
Hyponatremia may occur as a result of treatment with SSRIs, including escitalopram oxalate. In many cases, this hyponatremia appears to be the result of the syndrome of inappropriate antidiuretic hormone secretion (SIADH), and was reversible when escitalopram oxalate was discontinued. Cases with serum sodium lower than 110 mmol/L have been reported. Elderly patients may be at greater risk of developing hyponatremia with SSRIs and SNRIs. Also, patients taking diuretics or who are otherwise volume depleted may be at greater risk [ see Use in Specific Populations ( 8.5 )]. Consider discontinuation of escitalopram oxalate in patients with symptomatic hyponatremia and appropriate medical intervention should be instituted.
Signs and symptoms of hyponatremia include headache, difficulty concentrating, memory impairment, confusion, weakness, and unsteadiness, which may lead to falls. Signs and symptoms associated with more severe and/or acute cases have included hallucination, syncope, seizure, coma, respiratory arrest, and death.
^ Recommended Dosage For Specific Populations
The recommended dosage for most elderly patients and patients with hepatic impairment is 10 mg once daily [see Use in Specific Populations ( 8.5, 8.6)].
The recommended dosage for escitalopram tablets in adults with a creatinine clearance less than 20 mL/minute has not been determined. No dosage adjustment is necessary for patients with mild or moderate renal impairment [see Use in Specific Populations ( 8.7)].
^ Pharmacokinetics
The single- and multiple-dose pharmacokinetics of escitalopram are linear and dose-proportional in a dose range of 10 to 30 mg/day. With once-daily dosing, steady state plasma concentrations are achieved within approximately one week. At steady state, the extent of accumulation of escitalopram in plasma in young healthy subjects was 2.2 to 2.5 times the plasma concentrations observed after a single dose.
^ Angle Closure Glaucoma
The pupillary dilation that occurs following use of many antidepressant drugs including escitalopram oxalate may trigger an angle closure attack in a patient with anatomically narrow angles who does not have a patent iridectomy.
^ Seizures
Although anticonvulsant effects of racemic citalopram have been observed in animal studies, escitalopram oxalate has not been systematically evaluated in patients with a seizure disorder. These patients were excluded from clinical studies during the product's premarketing testing. In clinical trials of escitalopram oxalate, cases of convulsion have been reported in association with escitalopram oxalate treatment. Like other drugs effective in the treatment of major depressive disorder, escitalopram oxalate should be introduced with care in patients with a history of seizure disorder.
^ Suicidal Thoughts And Behaviors In Adolescents And Young Adults
In pooled analyses of placebo-controlled trials of antidepressant drugs (SSRIs and other antidepressant classes) that included approximately 77,000 adult patients and 4,500 pediatric patients, the incidence of suicidal thoughts and behaviors in the antidepressant-treated patients age 24 years and younger was greater than in placebo-treated patients. There was considerable variation in risk of suicidal thoughts and behaviors among drugs, but there was an increased risk identified in young patients for most drugs studied. There were differences in absolute risk of suicidal thoughts and behaviors across the different indications, with the highest incidence in patients with MDD. The drug-placebo differences in the number of cases of suicidal thoughts and behaviors per 1000 patients treated are provided in Table 1.
It is unknown whether the risk of suicidal thoughts and behaviors in children, adolescents, and young adults extends to longer-term use, i.e., beyond four months. However, there is substantial evidence from placebo-controlled maintenance trials in adults with MDD that antidepressants delay the recurrence of depression and that depression itself is a risk factor for suicidal thoughts and behaviors.
Monitor all antidepressant-treated patients for any indication for clinical worsening and emergence of suicidal thoughts and behaviors, especially during the initial few months of drug therapy, and at times of dosage changes. Counsel family members or caregivers of patients to monitor for changes in behavior and to alert the healthcare provider. Consider changing the therapeutic regimen, including possibly discontinuing escitalopram oxalate, in patients whose depression is persistently worse, or who are experiencing emergent suicidal thoughts or behaviors.
^ Discontinuation Of Treatment With Escitalopram Tablets
Symptoms associated with discontinuation of escitalopram tablets and other SSRIs and SNRIs have been reported [see Warnings and Precautions ( 5.3)]. Patients should be monitored for these symptoms when discontinuing treatment. A gradual reduction in the dose rather than abrupt cessation is recommended whenever possible. If intolerable symptoms occur following a decrease in the dose or upon discontinuation of treatment, then resuming the previously prescribed dose may be considered. Subsequently, the physician may continue decreasing the dose but at a more gradual rate.
^ Generalized Anxiety Disorder
Adults
The efficacy of escitalopram oxalate in the treatment of generalized anxiety disorder (GAD) in adults was demonstrated in three, 8-week, multicenter, flexible-dose, placebo-controlled studies that compared escitalopram oxalate (10 mg to 20 mg daily) to placebo in outpatients between 18 and 80 years of age who met DSM-IV criteria for GAD. In all three studies, escitalopram oxalate showed statistically significant greater mean improvement compared to placebo on the Hamilton Anxiety Scale (HAM-A).
There were too few patients in differing ethnic and age groups to adequately assess whether or not escitalopram oxalate has differential effects in these groups. There was no difference in response to escitalopram oxalate between men and women.
Additional pediatric use information is approved for AbbVie Inc.’s LEXAPRO (escitalopram) tablets and LEXAPRO (escitalopram) oral solution. However, due to AbbVie Inc.’s marketing exclusivity rights, this drug product is not labeled with that information.
^ Mechanism Of Action
The mechanism of antidepressant action of escitalopram, the S-enantiomer of racemic citalopram, is presumed to be linked to potentiation of serotonergic activity in the central nervous system (CNS) resulting from its inhibition of CNS neuronal reuptake of serotonin (5-HT).