^ Cardiovascular Outcomes In Adults With Type 2 Diabetes Mellitus And Atherosclerotic Cardiovascular Disease
The effect of JARDIANCE on cardiovascular (CV) risk in adult patients with type 2 diabetes mellitus and established, stable, atherosclerotic CV disease was evaluated in the EMPA-REG OUTCOME trial, a multicenter, multinational, randomized, double-blind parallel group trial. The trial compared the risk of experiencing a major adverse cardiovascular event (MACE) between JARDIANCE and placebo when these were added to and used concomitantly with standard of care treatments for diabetes mellitus and atherosclerotic CV disease. Concomitant antidiabetic medications were to be kept stable for the first 12 weeks of the trial. Thereafter, antidiabetic and atherosclerotic therapies could be adjusted, at the discretion of investigators, to ensure participants were treated according to the standard care for these diseases.
A total of 7,020 patients were treated (JARDIANCE 10 mg = 2,345; JARDIANCE 25 mg = 2,342; placebo = 2,333) and followed for a median of 3.1 years. Approximately 72% of the trial population was White, 22% was Asian, and 5% was Black. The mean age was 63 years and approximately 72% were male.
All patients in the trial had inadequately controlled type 2 diabetes mellitus at baseline (HbA1c greater than or equal to 7%). The mean HbA1c at baseline was 8.1% and 57% of participants had diabetes mellitus for more than 10 years. Approximately 31%, 22% and 20% reported a past history of neuropathy, retinopathy and nephropathy to investigators, respectively and the mean eGFR was 74 mL/min/1.73 m2. At baseline, patients were treated with one (~30%) or more (~70%) antidiabetic medications including metformin (74%), insulin (48%), and sulfonylurea (43%).
All patients had established atherosclerotic CV disease at baseline including one (82%) or more (18%) of the following: a documented history of coronary artery disease (76%), stroke (23%) or peripheral artery disease (21%). At baseline, the mean systolic blood pressure was 136 mmHg, the mean diastolic blood pressure was 76 mmHg, the mean LDL was 86 mg/dL, the mean HDL was 44 mg/dL, and the mean urinary albumin to creatinine ratio (UACR) was 175 mg/g. At baseline, approximately 81% of patients were treated with renin angiotensin system inhibitors, 65% with beta-blockers, 43% with diuretics, 77% with statins, and 86% with antiplatelet agents (mostly aspirin).
The primary endpoint in EMPA-REG OUTCOME was the time to first occurrence of a Major Adverse Cardiac Event (MACE). A major adverse cardiac event was defined as occurrence of either a CV death or a non-fatal myocardial infarction (MI) or a non-fatal stroke. The statistical analysis plan had pre-specified that the 10 and 25 mg doses would be combined. A Cox proportional hazards model was used to test for non-inferiority against the pre-specified risk margin of 1.3 for the hazard ratio of MACE and superiority on MACE if non-inferiority was demonstrated. Type-1 error was controlled across multiples tests using a hierarchical testing strategy.
JARDIANCE significantly reduced the risk of first occurrence of primary composite endpoint of CV death, non-fatal myocardial infarction, or non-fatal stroke (HR: 0.86; 95% CI: 0.74, 0.99). The treatment effect was due to a significant reduction in the risk of CV death in subjects randomized to empagliflozin (HR: 0.62; 95% CI: 0.49, 0.77), with no change in the risk of non-fatal myocardial infarction or non-fatal stroke (see Table 15 and Figures 5 and 6). Results for the 10 mg and 25 mg empagliflozin dosages were consistent with results for the combined dosage groups.
The efficacy of JARDIANCE on CV death was generally consistent across major demographic and disease subgroups.
Vital status was obtained for 99.2% of subjects in the trial. A total of 463 deaths were recorded during the EMPA-REG OUTCOME trial. Most of these deaths were categorized as CV deaths. The non-CV deaths were only a small proportion of deaths and were balanced between the treatment groups (2.1% in patients treated with JARDIANCE, and 2.4% of patients treated with placebo).
^1 Indications And Usage
JARDIANCE is indicated:
^7 Drug Interactions
See Table 4 for clinically relevant interactions with JARDIANCE.
^ Overdosage
In the event of an overdose with JARDIANCE, consider contacting the Poison Help line (1-800-222-1222) or a medical toxicologist for additional overdosage management recommendations. Removal of empagliflozin by hemodialysis has not been studied.
^ Chronic Kidney Disease Trial In Adults
EMPA-KIDNEY (NCT03594110) was a randomized, double-blind, placebo-controlled trial conducted in adults with chronic kidney disease (eGFR ≥20 to <45 mL/min/1.73 m2; or eGFR ≥45 to <90 mL/min/1.73 m2 with urine albumin to creatinine ratio [UACR] ≥200 mg/g). The trial excluded patients with polycystic kidney disease or patients requiring intravenous immunosuppressive therapy in the preceding three months or >45 mg of prednisone (or equivalent) at the time of screening. The primary objective of the trial was to assess the effects of empagliflozin as an adjunct to standard of care therapy, including RAS-inhibitor therapy when appropriate, on time to kidney disease progression or cardiovascular death.
A total of 6,609 patients, were equally randomized to JARDIANCE 10 mg or placebo and were followed for a median of 24 months.
The mean age of the study population was 63 years (range: 18 to 94 years) and 67% were male. Approximately 58% of the study population were White, 36% Asian, and 4% Black or African American. Approximately 44% of the patients had type 2 diabetes mellitus.
At baseline, the mean eGFR was 37 mL/min/1.73 m2, 21% of patients had an eGFR equal to or above 45 mL/min/1.73 m2, 44% had an eGFR 30 to less than 45 mL/min/1.73 m2, and 35% had an eGFR less than 30 mL/min/1.73 m2. The median UACR was 329 mg/g, 20% of patients had a UACR <30 mg/g, 28% had a UACR 30 to ≤300mg/g, and 52% had a UACR >300 mg/g. Approximately 1% of patients had type 1 diabetes at baseline. The most common etiologies of CKD were diabetic nephropathy/diabetic kidney disease (31%), glomerular disease (25%), hypertensive/renovascular disease (22%) and other/unknown (22%).
At baseline, 85% of patients were treated with ACE inhibitor or ARB, 64% with statins, and 34% with antiplatelet agents.
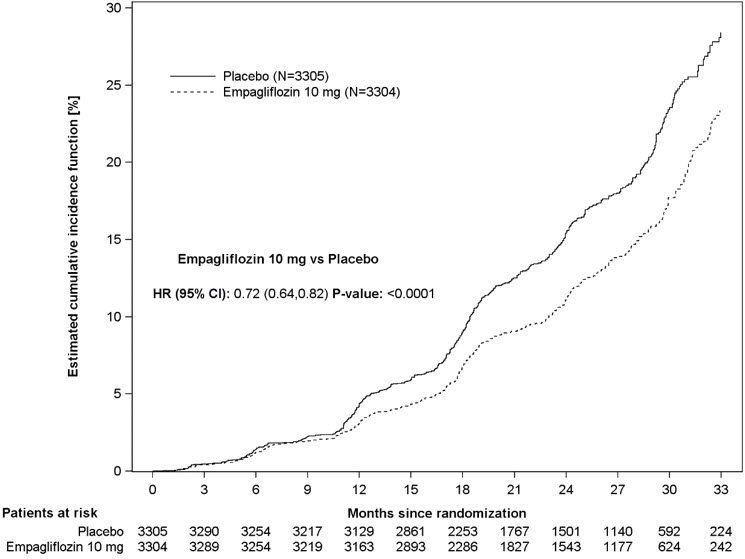
JARDIANCE was superior to placebo in reducing the risk of the primary composite endpoint of sustained ≥40% eGFR decline, sustained eGFR <10 mL/min/1.73 m2, progression to end-stage kidney disease, or CV or renal death. The treatment effect reflected a reduction in a sustained ≥40% eGFR decline, sustained eGFR <10 mL/min/1.73 m2, progression to end-stage kidney disease, and CV death. There were few renal deaths during the trial. JARDIANCE also reduced the risk of first and recurrent hospitalization (see Table 18 and Figure 13); information collected on the reason for hospitalization was insufficient to further characterize the benefit.
Figure 13 Time to First Occurrence of the Primary Composite Endpoint, Sustained ≥40% eGFR Decline, Sustained eGFR <10 mL/min/1.73 m2, ESKD or Renal Death, or CV Death
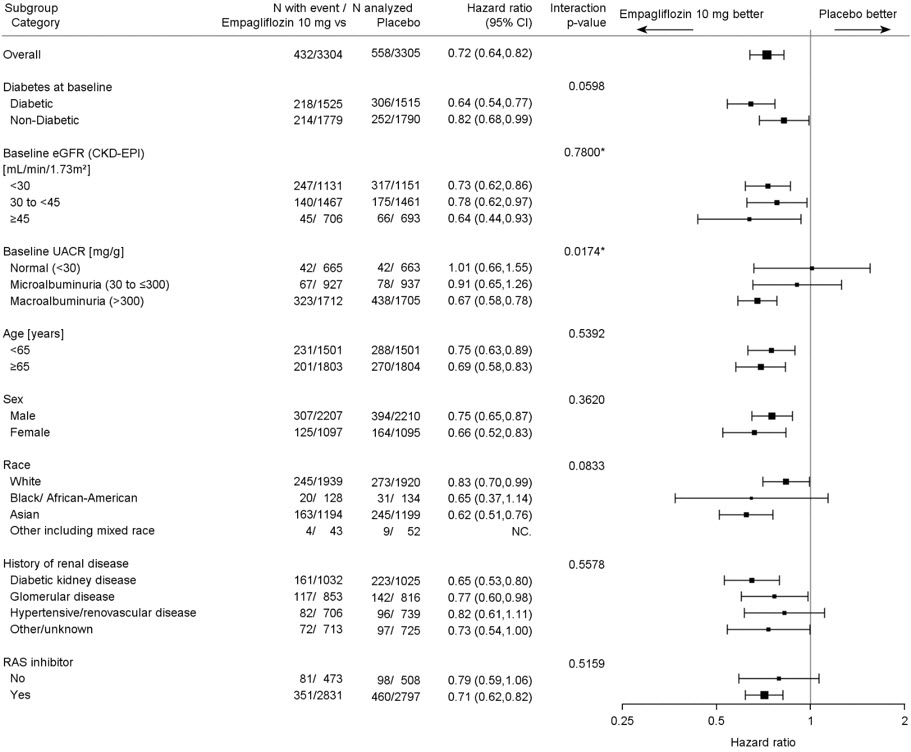
The results of the primary composite endpoint were generally consistent across the pre-specified subgroups examined, including eGFR categories, underlying cause of kidney disease, diabetes status, or background use of RAS inhibitors (see Figure 14). The treatment benefit with JARDIANCE on the primary composite endpoint was not evident in patients with very low levels of albuminuria, however there were few events in these patients.
Figure 14 Treatment Effects for the Primary Composite Endpoint (Sustained ≥40% eGFR Decline, Sustained eGFR <10 mL/min/1.73 m2, ESKD or Renal Death, or CV Death) Subgroup Analysis (EMPA-KIDNEY)
*=Trend test
^ Renal Impairment
The efficacy and safety of JARDIANCE for glycemic control were evaluated in a trial of adult patients with type 2 diabetes mellitus with mild and moderate renal impairment (eGFR 30 to less than 90 mL/min/1.73 m2) [see Clinical Studies (14.1)]. In this trial, 195 adult patients exposed to JARDIANCE had an eGFR between 60 and 90 mL/min/1.73 m2, 91 adult patients exposed to JARDIANCE had an eGFR between 45 and 60 mL/min/1.73 m2, and 97 patients exposed to JARDIANCE had an eGFR between 30 and 45 mL/min/1.73 m2. The glucose lowering benefit of JARDIANCE 25 mg decreased in adult patients with worsening renal function. The risks of renal impairment, volume depletion adverse reactions and urinary tract infection-related adverse reactions increased with worsening renal function [see Warnings and Precautions (5.2)]. Use of JARDIANCE for glycemic control in patients without established cardiovascular disease or cardiovascular risk factors is not recommended when eGFR is less than 30 mL/min/1.73 m2.
JARDIANCE was evaluated in 7,020 adult patients with type 2 diabetes and established cardiovascular disease (eGFR greater than or equal to 30 mL/min/1.73 m2) in the EMPA-REG OUTCOME trial, in a total of 9,718 patients with heart failure (eGFR greater than or equal to 20 mL/min/1.73 m2) in the EMPEROR-Reduced and EMPEROR-Preserved trials, and in 6,609 adult patients with chronic kidney disease (eGFR 20 to 90 mL/min/1.73 m2) in the EMPA-KIDNEY study. The safety profile across eGFR subgroups in these trials was consistent with the known safety profile [see Adverse Reactions (6.1) and Clinical Studies (14.3, 14.4, 14.5)].
Efficacy and safety trials with JARDIANCE did not enroll adult patients with an eGFR less than 20 mL/min/1.73 m2 or on dialysis. Once enrolled, adult patients in the EMPA-REG OUTCOME, EMPEROR-Reduced, EMPEROR-Preserved, and EMPA-KIDNEY trials were not required to discontinue therapy for worsening of eGFR to less than 20 mL/min/1.73 m2 or initiation of dialysis [see Clinical Studies (14.3, 14.4, 14.5)].
^3 Dosage Forms And Strengths
JARDIANCE tablets available as:
^ Pediatric Use
The safety and effectiveness of JARDIANCE as an adjunct to diet and exercise to improve glycemic control in type 2 diabetes mellitus have been established in pediatric patients aged 10 years and older. Use of JARDIANCE for this indication is supported by evidence from a 26-week double-blind, placebo-controlled clinical trial, with a double-blind active treatment safety extension period of up to 52 weeks in 157 pediatric patients aged 10 to 17 years with type 2 diabetes mellitus and a pediatric pharmacokinetic study [see Clinical Pharmacology (12.3) and Clinical Studies (14.2)]. The safety profile of pediatric patients treated with JARDIANCE was similar to that observed in adults with type 2 diabetes mellitus, with the exception of hypoglycemia risk which was higher in pediatric patients treated with JARDIANCE regardless of concomitant insulin use [see Warnings and Precautions (5.4) and Adverse Reactions (6.1)].
The safety and effectiveness of JARDIANCE have not been established in pediatric patients less than 10 years of age as an adjunct to diet and exercise to improve glycemic control in type 2 diabetes mellitus.
The safety and effectiveness of JARDIANCE have not been established in pediatric patients to reduce the risk of:
^ Recommendations Regarding Missed Dose
^ Geriatric Use
In glycemic control trials in patients with type 2 diabetes mellitus, a total of 2,721 (32%) patients treated with JARDIANCE were 65 years of age and older, and 491 (6%) were 75 years of age and older. JARDIANCE is expected to have diminished glycemic efficacy in elderly patients with renal impairment [see Use in Specific Populations (8.6)]. The risk of volume depletion-related adverse reactions increased in patients who were 75 years of age and older to 2.1%, 2.3%, and 4.4% for placebo, JARDIANCE 10 mg, and JARDIANCE 25 mg. The risk of urinary tract infections increased in patients who were 75 years of age and older to 10.5%, 15.7%, and 15.1% in patients randomized to placebo, JARDIANCE 10 mg, and JARDIANCE 25 mg, respectively [see Warnings and Precautions (5.2) and Adverse Reactions (6.1)].
In the EMPEROR-Reduced, EMPEROR-Preserved, and EMPA-KIDNEY trials, no overall differences in safety and effectiveness have been observed between patients 65 years of age and older and younger adult patients. EMPEROR-Reduced included 1,188 (64%) patients treated with JARDIANCE 65 years of age and older, and 503 (27%) patients 75 years of age and older. EMPEROR-Preserved included 2,402 (80%) patients treated with JARDIANCE 65 years of age and older, and 1,281 (43%) patients 75 years of age and older. EMPA-KIDNEY included 2,089 (32%) patients treated with JARDIANCE 65 years of age and older, and 1,518 (23%) patients 75 years of age and older.
^ Recommended Dosage
Table 1 presents the recommended dosage of JARDIANCE in adult and pediatric patients aged 10 years and older.
^ Temporary Interruption For Surgery
Withhold JARDIANCE for at least 3 days, if possible, prior to major surgery or procedures associated with prolonged fasting. Resume JARDIANCE when the patient is clinically stable and has resumed oral intake [see Warnings and Precautions (5.1) and Clinical Pharmacology (12.2) ].
^ Hypoglycemia
Insulin and insulin secretagogues are known to cause hypoglycemia. In adult patients, the risk of hypoglycemia may be increased when JARDIANCE is used in combination with insulin secretagogues (e.g., sulfonylurea) or insulin. In pediatric patients aged 10 years and older, the risk of hypoglycemia was higher with JARDIANCE regardless of insulin use [see Adverse Reactions (6.1) ] .
The risk of hypoglycemia may be lowered by a reduction in the dose of sulfonylurea (or other concomitantly administered insulin secretagogues) or insulin. Inform patients using these concomitant medications and pediatric patients of the risk of hypoglycemia and educate them on the signs and symptoms of hypoglycemia.
^ Hepatic Impairment
JARDIANCE may be used in patients with hepatic impairment [see Clinical Pharmacology (12.3)].
^ Pharmacokinetics
The pharmacokinetics of empagliflozin has been characterized in healthy volunteers and patients with type 2 diabetes mellitus and no clinically relevant differences were noted between the two populations. The steady-state mean plasma AUC and Cmax were 1,870 nmol∙h/L and 259 nmol/L, respectively, with 10 mg empagliflozin once daily treatment, and 4,740 nmol∙h/L and 687 nmol/L, respectively, with 25 mg empagliflozin once daily treatment. Systemic exposure of empagliflozin increased in a dose-proportional manner in the therapeutic dose range. Empagliflozin does not appear to have time-dependent pharmacokinetic characteristics. Following once-daily dosing, up to 22% accumulation, with respect to plasma AUC, was observed at steady-state.
^ Necrotizing Fasciitis Of The Perineum (fournier's Gangrene)
Reports of necrotizing fasciitis of the perineum (Fournier's gangrene), a rare but serious and life-threatening necrotizing infection requiring urgent surgical intervention, have been identified in patients with diabetes mellitus receiving SGLT2 inhibitors, including JARDIANCE. Cases have been reported in both females and males. Serious outcomes have included hospitalization, multiple surgeries, and death.
Patients treated with JARDIANCE presenting with pain or tenderness, erythema, or swelling in the genital or perineal area, along with fever or malaise, should be assessed for necrotizing fasciitis. If suspected, start treatment immediately with broad-spectrum antibiotics and, if necessary, surgical debridement. Discontinue JARDIANCE, closely monitor blood glucose levels, and provide appropriate alternative therapy for glycemic control.
^ Lower Limb Amputation
In some clinical studies with SGLT2 inhibitors an imbalance in the incidence of lower limb amputation has been observed. Across four JARDIANCE outcome trials, lower limb amputation event rates were 4.3 and 5.0 events per 1,000 patient-years in the placebo group and the JARDIANCE 10 mg or 25 mg dose group, respectively, with a HR of 1.05 (95 % CI) (0.81, 1.36).
In a long-term cardio-renal outcome trial [see Clinical Studies 14.5 ] , in patients with chronic kidney disease, the occurrence of lower limb amputations was reported with event rates of 2.9, and 4.3 events per 1000 patient-years in the placebo, and JARDIANCE 10 mg treatment arms, respectively. Amputation of the toe and mid-foot were most frequent (21 out of 28 JARDIANCE 10 mg treated patients with lower limb amputations), and some involving above and below the knee. Some patients had multiple amputations.
Peripheral artery disease, and diabetic foot infection (including osteomyelitis), were the most common precipitating medical events leading to the need for an amputation. The risk of amputation was highest in patients with a baseline history of diabetic foot, peripheral artery disease (including previous amputation) or diabetes.
Counsel patients about the importance of routine preventative foot care. Monitor patients receiving JARDIANCE for signs and symptoms of diabetic foot infection (including osteomyelitis), new pain or tenderness, sores or ulcers involving the lower limbs, and institute appropriate treatment.
^ Glycemic Control Trial In Pediatric Patients Aged To Years With Type 2 Diabetes Mellitus
DINAMO (NCT03429543) was a 26-week, double-blind, randomized, placebo-controlled, parallel group trial, with a double-blind active treatment safety extension period up to 52 weeks to assess the efficacy of JARDIANCE. The trial enrolled pediatric patients aged 10 to 17 years with inadequately controlled type 2 diabetes mellitus (HbA1c 6.5 to 10.5%). Patients treated with metformin (at least 1,000 mg daily or maximally tolerated dose), with or without insulin therapy, and those with a history of intolerance to metformin therapy were enrolled. Patients were randomized to 3 treatment arms (JARDIANCE 10 mg, a dipeptidyl peptidase-4 (DPP-4) inhibitor or placebo), over 26 weeks. Patients in the JARDIANCE 10 mg group who failed to achieve HbA1c <7.0% at Week 12 underwent a second randomization at Week 14 to remain on the 10 mg dose or increase to 25 mg. Patients on placebo were re-randomized at Week 26 to one of the JARDIANCE doses (10 mg or 25 mg) or a DPP-4 inhibitor.
A total of 157 patients were treated with either JARDIANCE (10 mg or 25 mg; N=52), a DPP-4 inhibitor (N=52), or placebo (N=53). Background therapies as adjunct to diet and exercise included metformin (51%), a combination of metformin and insulin (40.1%), insulin (3.2%), or none (5.7%). The mean HbA1c at baseline was 8.0% and the mean duration of type 2 diabetes mellitus was 2.1 years. The mean age was 14.5 years (range: 10-17 years) and 51.6% were aged 15 years and older. Approximately, 50% were White, 6% were Asian, 31% were Black or African American, and 38% were of Hispanic or Latino ethnicity. The mean BMI was 36.0 kg/m2 and mean BMI Z-score was 3.0. Patients with an eGFR less than 60 mL/min/1.73 m2 were not enrolled in the trial. Approximately 25% of the study population had microalbuminuria or macroalbuminuria.
At Week 26, treatment with JARDIANCE was superior in reducing HbA1c from baseline versus placebo (see Table 14).
^ Testing Prior To Initiation Of Jardiance
^ Heart Failure Trials In Adults
EMPEROR-Reduced (NCT03057977) was a double-blind trial conducted in adults with chronic heart failure (New York Heart Association [NYHA] functional class II-IV) with left ventricular ejection fraction (LVEF) ≤40% to evaluate the efficacy of JARDIANCE as adjunct to standard of care heart failure therapy.
Of 3,730 patients, 1,863 were randomized to JARDIANCE 10 mg and 1,867 to placebo and were followed for a median of 16 months. The mean age of the trial population was 67 years (range: 25 to 94 years) and 76% were men, 24% were women, and 27% were 75 years of age or older. Approximately 71% of the trial population were White, 18% Asian, and 7% Black or African American. At baseline, 50% of the patients had type 2 diabetes mellitus.
At randomization, 75% of patients were NYHA class II, 24% were class III, and 0.5% were class IV. The mean LVEF was 28%. At baseline, the mean eGFR was 62 mL/min/1.73 m2 and the median urinary albumin to creatinine ratio (UACR) was 22 mg/g. Approximately half of the patients (52%) had eGFR equal to or above 60 mL/min/1.73 m2, 24% had eGFR 45 to less than 60 mL/min/1.73 m2, 19% had eGFR 30 to less than 45 mL/min/1.73 m2, and 5% had eGFR 20 to less than 30 mL/min/1.73 m2.
At baseline, 88% of patients were treated with angiotensin-converting enzyme (ACE) inhibitors, angiotensin receptor blockers (ARB), or angiotensin receptor-neprilysin inhibitors (ARNI), 95% with beta-blockers, 71% with mineralocorticoid receptor antagonists (MRA), and 95% with diuretics.
The primary endpoint was the time to first event of either cardiovascular (CV) death or hospitalization for heart failure (HHF). First and recurrent HHF was assessed as a key secondary endpoint.
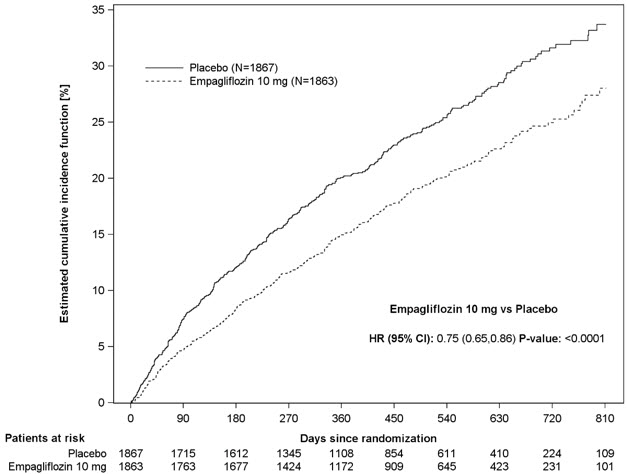
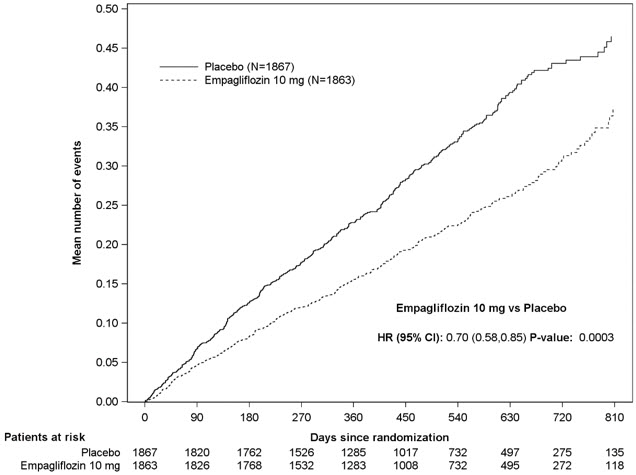
JARDIANCE was superior in reducing the risk of the primary composite endpoint of cardiovascular death or hospitalization for heart failure compared with placebo, mostly through a reduction in hospitalization for heart failure. JARDIANCE reduced the risk of first and recurrent HHF (see Table 16 and Figures 7 and 8).
Figure 7 Time to First Occurrence of the Primary Composite Endpoint of CV Death or Hospitalization for Heart Failure
Figure 8 Time to Event of Hospitalization for Heart Failure (First and Recurrent)
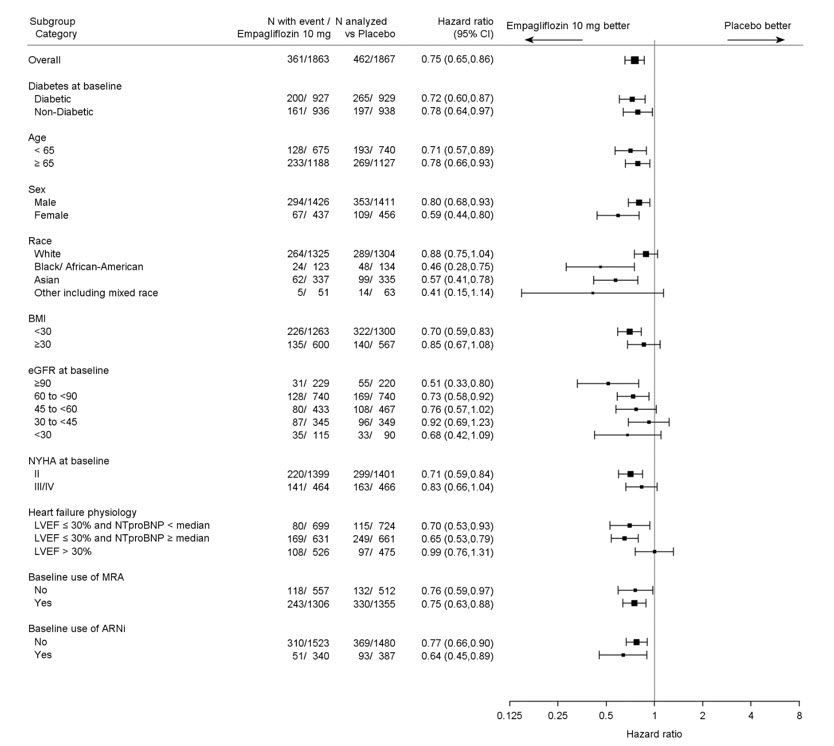
The results of the primary composite were generally consistent across the pre-specified subgroups (see Figure 9).
Figure 9 Treatment Effects for the Primary Composite Endpoint (CV Death and Hospitalization for Heart Failure) Subgroup Analysis (EMPEROR-Reduced)
LVEF >30%: Includes both above and below the median NT-proBNP. To be eligible for inclusion, patients with an LVEF >30% were required to meet a higher NT-proBNP threshold than those with LVEF ≤30%, unless they additionally had a history of HHF within the past 12 months.
EMPEROR-Preserved (NCT03057951) was a double-blind trial conducted in adults with chronic heart failure NYHA Class II-IV with LVEF >40% to evaluate the efficacy of JARDIANCE as adjunct to standard of care therapy.
Of 5,988 patients, 2,997 were randomized to JARDIANCE 10 mg and 2,991 to placebo and were followed for a median of 26 months. The mean age of the trial population was 72 years (range: 22 to 100 years) and 55% were men, 45% were women, and 43% were 75 years of age or older. Approximately 76% of the trial population were White, 14% Asian, and 4% Black or African American.
At randomization, 82% of patients were NYHA class II, 18% were class III, and 0.3% were class IV. The EMPEROR-Preserved trial population included patients with a LVEF <50% (33.1%), with a LVEF 50 to <60% (34.4%) and a LVEF ≥60% (32.5%). At baseline, the mean eGFR was 61 mL/min/1.73 m2 and the median urinary albumin to creatinine ratio (UACR) was 21 mg/g. Approximately half of the patients (50%) had eGFR equal to or above 60 mL/min/1.73 m2, 26% had eGFR 45 to less than 60 mL/min/1.73 m2, 19% had eGFR 30 to less than 45 mL/min/1.73 m2, and 5% had eGFR 20 to less than 30 mL/min/1.73 m2.
At baseline, 81% of patients were treated with ACE inhibitors, ARBs, or ARNI, 86% with beta-blockers, 38% with MRAs, and 86% with diuretics.
The primary endpoint was the time to first event of either CV death or HHF. First and recurrent HHF was assessed as a key secondary endpoint.
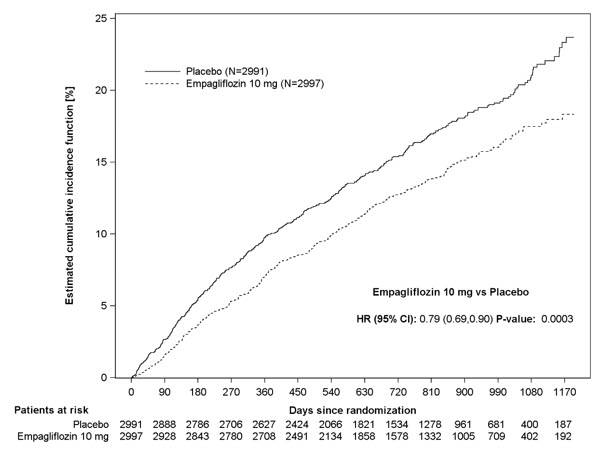
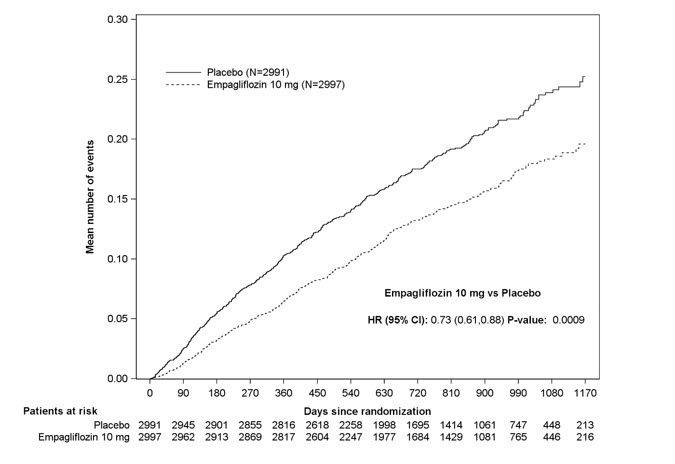
JARDIANCE was superior in reducing the risk of the primary composite endpoint compared with placebo, mostly through a reduction in hospitalization for heart failure. JARDIANCE reduced the risk of first and recurrent HHF (see Table 17 and Figures 10 and 11).
Figure 10 Time to First Occurrence of the Primary Composite Endpoint of CV Death or Hospitalization for Heart Failure
Figure 11 Time to Event of Hospitalization for Heart Failure (First and Recurrent)
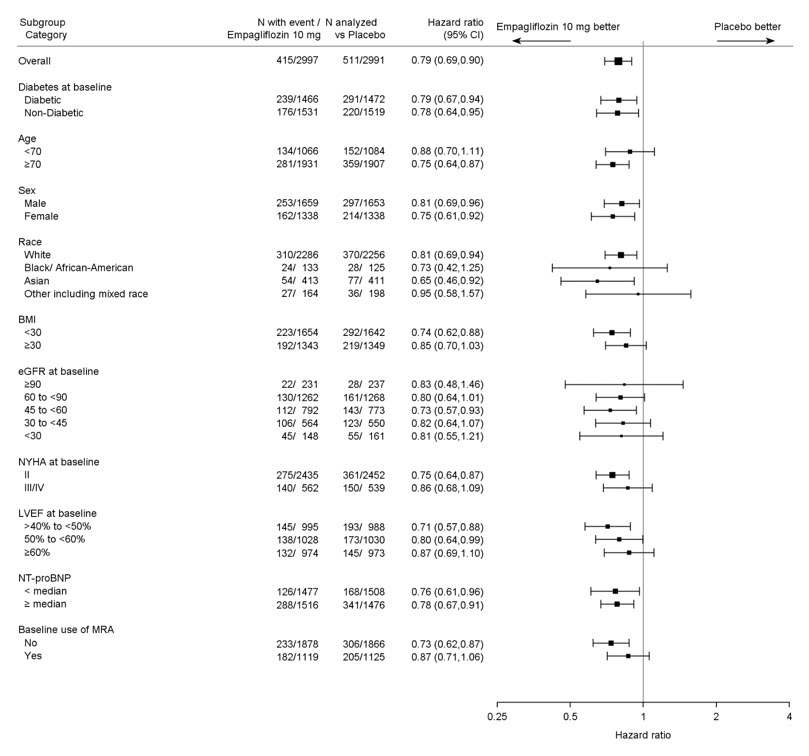
The results of the primary composite endpoint were consistent across the pre-specified subgroups (see Figure 12).
Figure 12 Treatment Effects for the Primary Composite Endpoint (CV Death or Hospitalization for Heart Failure) Subgroup Analysis (EMPEROR-Preserved)
^ Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide).
^ Glycemic Control Trials In Adults With Type 2 Diabetes Mellitus
JARDIANCE has been studied as monotherapy and in combination with metformin, sulfonylurea, pioglitazone, linagliptin, and insulin. JARDIANCE has also been studied in patients with type 2 diabetes mellitus with mild or moderate renal impairment.
In adult patients with type 2 diabetes mellitus, treatment with JARDIANCE reduced hemoglobin A1c (HbA1c), compared to placebo. The reduction in HbA1c for JARDIANCE compared with placebo was observed across subgroups including sex, race, geographic region, baseline BMI and duration of disease.
^6 Adverse Reactions
The following important adverse reactions are described below and elsewhere in the labeling:
^ Mechanism Of Action
Empagliflozin is an inhibitor of SGLT2, the predominant transporter responsible for reabsorption of glucose from the glomerular filtrate back into the circulation. By inhibiting SGLT2, empagliflozin reduces renal reabsorption of filtered glucose and lowers the renal threshold for glucose, and thereby increases urinary glucose excretion.
Empagliflozin also reduces sodium reabsorption and increases the delivery of sodium to the distal tubule. This may influence several physiological functions including, but not restricted to, increasing tubuloglomerular feedback and reducing intraglomerular pressure, lowering both pre- and afterload of the heart and downregulating sympathetic activity.
^ Hypersensitivity Reactions
There have been postmarketing reports of serious hypersensitivity reactions (e.g., angioedema) in patients treated with JARDIANCE. If a hypersensitivity reaction occurs, discontinue JARDIANCE; treat promptly per standard of care, and monitor until signs and symptoms resolve. JARDIANCE is contraindicated in patients with hypersensitivity to empagliflozin or any of the excipients in JARDIANCE [see Contraindications (4)].
^ Genital Mycotic Infections
JARDIANCE increases the risk for genital mycotic infections [see Adverse Reactions (6.1)]. Patients with a history of chronic or recurrent genital mycotic infections were more likely to develop genital mycotic infections. Monitor and treat as appropriate.
^ Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
JARDIANCE has been evaluated in clinical trials in adult and pediatric patients aged 10 to 17 years with type 2 diabetes mellitus, in adults with heart failure, and in adults with chronic kidney disease. The overall safety profile of JARDIANCE was generally consistent across the studied indications.
^ Description
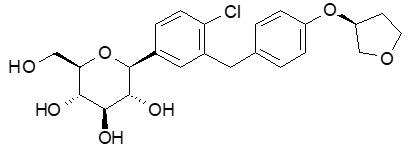
JARDIANCE tablets for oral use contain empagliflozin, an inhibitor of the SGLT2.
The chemical name of empagliflozin is D-Glucitol,1,5-anhydro-1-C-[4-chloro-3-[[4-[[(3S)-tetrahydro-3-furanyl]oxy]phenyl]methyl]phenyl]-, (1S).
Its molecular formula is C23H27ClO7 and the molecular weight is 450.91. The structural formula is:
Empagliflozin is a white to yellowish, non-hygroscopic powder. It is very slightly soluble in water, sparingly soluble in methanol, slightly soluble in ethanol and acetonitrile, soluble in 50% acetonitrile/water, and practically insoluble in toluene.
Each film-coated tablet of JARDIANCE contains 10 mg or 25 mg of empagliflozin (free base) and the following inactive ingredients: colloidal silicon dioxide, croscarmellose sodium, hydroxypropyl cellulose, lactose monohydrate, magnesium stearate, and microcrystalline cellulose. In addition, the film coating contains the following inactive ingredients: hypromellose, polyethylene glycol, talc, titanium dioxide, and yellow ferric oxide.
^4 Contraindications
JARDIANCE is contraindicated in patients:
^ Diabetic Ketoacidosis In Patients With Type 1 Diabetes Mellitus And Other Ketoacidosis
In patients with type 1 diabetes mellitus, JARDIANCE significantly increases the risk of diabetic ketoacidosis, a life-threatening event, beyond the background rate. In placebo-controlled trials of patients with type 1 diabetes mellitus, the risk of ketoacidosis was markedly increased in patients who received sodium glucose co-transporter 2 (SGLT2) inhibitors compared to patients who received placebo and fatal ketoacidosis has occurred with JARDIANCE. JARDIANCE is not indicated for glycemic control in patients with type 1 diabetes mellitus.
Type 2 diabetes mellitus and pancreatic disorders (e.g., history of pancreatitis or pancreatic surgery) are also risk factors for ketoacidosis. There have been postmarketing reports of fatal events of ketoacidosis in patients with type 2 diabetes mellitus using SGLT2 inhibitors, including JARDIANCE.
Precipitating conditions for diabetic ketoacidosis or other ketoacidosis include under-insulinization due to insulin dose reduction or missed insulin doses, acute febrile illness, reduced caloric intake, ketogenic diet, surgery, volume depletion, and alcohol abuse.
Signs and symptoms are consistent with dehydration and severe metabolic acidosis and include nausea, vomiting, abdominal pain, generalized malaise, and shortness of breath. Blood glucose levels at presentation may be below those typically expected for diabetic ketoacidosis (e.g., less than 250 mg/dL). Ketoacidosis and glucosuria may persist longer than typically expected. Urinary glucose excretion persists for 3 days after discontinuing JARDIANCE [see Clinical Pharmacology (12.2) ] ; however, there have been postmarketing reports of ketoacidosis and/or glucosuria lasting greater than 6 days and some up to 2 weeks after discontinuation of SGLT2 inhibitors.
Consider ketone monitoring in patients with type 1 diabetes mellitus and consider ketone monitoring in others at risk for ketoacidosis if indicated by the clinical situation. Assess for ketoacidosis regardless of presenting blood glucose levels in patients who present with signs and symptoms consistent with severe metabolic acidosis. If ketoacidosis is suspected, discontinue JARDIANCE, promptly evaluate, and treat ketoacidosis, if confirmed. Monitor patients for resolution of ketoacidosis before restarting JARDIANCE.
Withhold JARDIANCE, if possible, in temporary clinical situations that could predispose patients to ketoacidosis. Resume JARDIANCE when the patient is clinically stable and has resumed oral intake [see Dosage and Administration (2.4) ] .
Educate all patients on the signs and symptoms of ketoacidosis and instruct patients to discontinue JARDIANCE and seek medical attention immediately if signs and symptoms occur.
^ Postmarketing Experience
Additional adverse reactions have been identified during postapproval use of JARDIANCE. Because these reactions are reported voluntarily from a population of uncertain size, it is generally not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Gastrointestinal Disorders: Constipation Infections: Necrotizing fasciitis of the perineum (Fournier's gangrene), urosepsis and pyelonephritis Metabolism and Nutrition Disorders: Ketoacidosis Renal and Urinary Disorders: Acute kidney injury Skin and Subcutaneous Tissue Disorders: Angioedema, skin reactions (e.g., rash, urticaria)
^ How Supplied/storage And Handling
JARDIANCE tablets are available as follows:
10 mg tablets: pale yellow, round, biconvex, and bevel-edged film-coated tablets debossed with "S 10" on one side and the Boehringer Ingelheim company symbol on the other side.
Bottles of approximately 720 film-coated tablets, NDC 55154-0411-8
25 mg tablets: pale yellow, oval, biconvex film-coated tablets, debossed with "S 25" on one side and the Boehringer Ingelheim company symbol on the other side.
Bottles of approximately 990 film-coated tablets, NDC 55154-0412-8
Dispense in a well-closed container as defined in the USP.
^Package/label Display Panel
Contains Approximately
990 Tablets
JARDIANCE®
(empagliflozin) tablets
25 mg
Rx Only
WARNING: Keep out of reach of
children.

^ Volume Depletion
JARDIANCE can cause intravascular volume depletion which may sometimes manifest as symptomatic hypotension or acute transient changes in creatinine [see Adverse Reactions (6.1)]. There have been post-marketing reports of acute kidney injury, some requiring hospitalization and dialysis, in patients with type 2 diabetes mellitus receiving SGLT2 inhibitors, including JARDIANCE. Patients with impaired renal function (eGFR less than 60 mL/min/1.73 m2), elderly patients, or patients on loop diuretics may be at increased risk for volume depletion or hypotension. Before initiating JARDIANCE in patients with one or more of these characteristics, assess volume status and renal function. In patients with volume depletion, correct this condition before initiating JARDIANCE. Monitor for signs and symptoms of volume depletion, and renal function after initiating therapy.
^ Urosepsis And Pyelonephritis
There have been reports of serious urinary tract infections including urosepsis and pyelonephritis requiring hospitalization in patients receiving JARDIANCE. Treatment with JARDIANCE increases the risk for urinary tract infections. Evaluate patients for signs and symptoms of urinary tract infections and treat promptly, if indicated [see Adverse Reactions (6) ].