^ Drug Reaction With Eosinophilia And Systemic Symptoms (dress)
Drug Reaction with Eosinophilia and Systemic Symptoms (DRESS) has been reported in patients taking NSAIDs such as diclofenac sodium. Some of these events have been fatal or life-threatening. DRESS typically, although not exclusively, presents with fever, rash, lymphadenopathy, and/or facial swelling. Other clinical manifestations may include hepatitis, nephritis, hematological abnormalities, myocarditis, or myositis. Sometimes symptoms of DRESS may resemble an acute viral infection. Eosinophilia is often present. Because this disorder is variable in its presentation, other organ systems not noted here may be involved. It is important to note that early manifestations of hypersensitivity, such as fever or lymphadenopathy, may be present even though rash is not evident. If such signs or symptoms are present, discontinue diclofenac sodium and evaluate the patient immediately.
^Uses
for the temporary relief of minor aches and pains of muscles and joints associated with simple backache, arthritis, strains and sprains
^ Anaphylactic Reactions
Diclofenac has been associated with anaphylactic reactions in patients with and without known hypersensitivity to diclofenac and in patients with aspirin-sensitive asthma [ see Contraindications (4)and Warnings and Precautions (5.8) ].
Seek emergency help if an anaphylactic reaction occurs.
^ Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Medication Guide) and Instructions for Use that accompanies each prescription dispensed. Inform patients, families, or their caregivers of the following information before initiating therapy with diclofenac sodium and periodically during the course of ongoing therapy.
^Directions
^ Description
Diclofenac sodium topical solution USP, 1.5% is a nonsteroidal anti-inflammatory drug, available as a clear, colorless to faintly pink-orange solution for topical application.
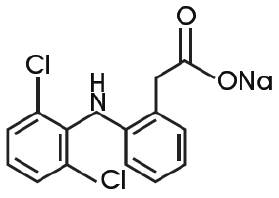
Diclofenac sodium topical solution contains 1.5% w/w diclofenac sodium, a benzeneacetic acid derivative that is a nonsteroidal anti-inflammatory drug (NSAID), designated chemically as 2-[(2,6-dichlorophenyl)amino]-benzeneacetic acid, monosodium salt. The molecular weight is 318.14. Its molecular formula is C 14H 10Cl 2NNaO 2and it has the following structural formula:
Each 1 mL of solution contains 16.05 mg of diclofenac sodium. In addition, diclofenac sodium topical solution contains the following inactive ingredients: dimethyl sulfoxide USP (DMSO, 45.5% w/w), ethanol, glycerin, propylene glycol and purified water.
^Instructions For Use Diclofenac Sodium Topical Solution
Read the Medication Guide that comes with diclofenac sodium topical solution first. Be sure that you read, understand and follow these Instructions for Use before you use diclofenac sodium topical solution for the first time.
Important: For use on the skin only (topical). Do not get diclofenac sodium topical solution in your eyes, nose or mouth.
Before you use Diclofenac Sodium Topical Solution:
Steps for using Diclofenac Sodium Topical Solution:
Step 1. Wash your hands with soap and water before applying diclofenac sodium topical solution.
Step 2. Put 10 drops of diclofenac sodium topical solution eitheron your hand ordirectly on your knee (see Figure A ).
Step 3. Spread diclofenac sodium topical solution evenly on the front, back and sides of your knee (see Figures B and C ). Repeat steps 2 and 3, three times so that your knee is completely covered with a totalof 40 drops of diclofenac sodium topical solution.
Step 4. If your healthcare provider has prescribed diclofenac sodium topical solution for both knees, repeat steps 2 and 3 for the other knee.
After you use Diclofenac Sodium Topical Solution:
Do not:
How should I store Diclofenac Sodium Topical Solution?
Keep diclofenac sodium topical solution and all medicines out of the reach of children.
This Instructions for Use has been approved by the U.S. Food and Drug Administration. Manufactured by: Taro Pharmaceuticals Inc., Brampton, Ontario, Canada L6T 1C1 Distributed by: Taro Pharmaceuticals U.S.A., Inc., Hawthorne, NY 10532
Revised: June 2021 5213715 57
^ Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The data described below reflect exposure to diclofenac sodium topical solution of 911 patients treated between 4 and 12 weeks (mean duration of 49 days) in seven Phase 3 controlled trials, as well as exposure of 793 patients treated in an open-label study, including 463 patients treated for at least 6 months, and 144 patients treated for at least 12 months. The population mean age was approximately 60 years, 89% of patients were Caucasians, 64% were females, and all patients had primary osteoarthritis. The most common adverse events with diclofenac sodium topical solution were application site skin reactions. These events were the most common reason for withdrawing from the studies.
^ Overdosage
Symptoms following acute NSAID overdosages have been typically limited to lethargy, drowsiness, nausea, vomiting, and epigastric pain, which have been generally reversible with supportive care. Gastrointestinal bleeding has occurred. Hypertension, acute renal failure, respiratory depression, and coma have occurred, but were rare. [ see Warnings and Precautions (5.1, 5.2. 5.4, 5.6) ].
Manage patients with symptomatic and supportive care following an NSAID overdosage. There are no specific antidotes. Emesis is not recommended due to a possibility of aspiration and subsequent respiratory irritation by DMSO contained in diclofenac sodium. Consider activated charcoal (60 to 100 grams in adults, 1 to 2 grams per kg of body weight in pediatric patients) and/or osmotic cathartic in symptomatic patients seen within four hours of ingestion or in patients with a large overdosage (5 to 10 times the recommended dosage). Forced diuresis, alkalinization of urine, hemodialysis, or hemoperfusion may not be useful due to high protein binding.
For additional information about overdose treatment, contact a poison control center (1-800-222-1222).
^ Hematologic Toxicity
Anemia has occurred in NSAID-treated patients. This may be due to occult or gross blood loss, fluid retention, or an incompletely described effect on erythropoiesis. If a patient treated with diclofenac sodium has any signs or symptoms of anemia, monitor hemoglobin or hematocrit. NSAIDs, including diclofenac sodium, may increase the risk of bleeding events. Co-morbid conditions such as coagulation disorders, concomitant use of warfarin, other anticoagulants, antiplatelet agents (e.g., aspirin), serotonin reuptake inhibitors (SSRIs) and serotonin norepinephrine reuptake inhibitors (SNRIs) may increase this risk. Monitor these patients for signs of bleeding [ see Drug Interactions (7) ].
The effects of diclofenac sodium on platelet function were studied in 10 healthy subjects administered 80 drops four times a day for 7 days. There was no significant change in platelet aggregation following one week of treatment [ see Clinical Pharmacology (12) ].
^3 Dosage Forms And Strengths
Diclofenac sodium topical solution USP, 1.5% w/w
^Keep Out Of Reach Of Children And Pets.
If swallowed, get medical help or contact a Poison Control Center right away. If inhaled, remove to fresh air. If breathing is difficult, get medical attention immediately.
^ Heart Failure And Edema
The Coxib and traditional NSAID Trialists' Collaboration meta-analysis of randomized controlled trials demonstrated an approximately two-fold increase in hospitalizations for heart failure in COX-2 selective-treated patients and nonselective NSAID-treated patients compared to placebo-treated patients. In a Danish National Registry study of patients with heart failure, NSAID use increased the risk of MI, hospitalization for heart failure, and death.
Additionally, fluid retention and edema have been observed in some patients treated with NSAIDs. Use of diclofenac may blunt the CV effects of several therapeutic agents used to treat these medical conditions (e.g., diuretics, ACE inhibitors, or angiotensin receptor blockers [ARBs]) [ see Drug Interactions (7) ].
Avoid the use of diclofenac sodium in patients with severe heart failure unless the benefits are expected to outweigh the risk of worsening heart failure. If diclofenac sodium is used in patients with severe heart failure, monitor patients for signs of worsening heart failure.
^ Masking Of Inflammation And Fever
The pharmacological activity of diclofenac sodium in reducing inflammation, and possibly fever, may diminish the utility of diagnostic signs in detecting infections.
^ Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
^4 Contraindications
Diclofenac sodium topical solution, USP is contraindicated in the following patients:
^Inactive Ingredients
Acrylates Copolymer, Acrylates/C10-30 Alkyl Acrylate Crosspolymer, Aloe Barbadensis (Aloe Vera) Leaf Juice, Aqua (Purified Water), Arnica Montana Flower Extract, Ethyl Alcohol, Ethyl Menthane Carboxamide, Euterpe Oleracea (Acai) Fruit Oil, Glycerin,Hydrated Silica, Hydrolyzed Corn Starch, Hydrolyzed Corn Starch Octenylsuccinate, Lauryl Laurate, Linum Usitatissimum (Linseed) Seed Oil, Mentha Piperita (Peppermint) Oil, Menthyl Lactate, Methyl Diisopropyl Propionamide, Phenoxyethanol, PPG-2 Hydroxyethyl Cocamide, Rosmarinus Officinals (Rosemary) Oil, Triethanolamine, Zea Mays (Corn) Starch.
^Lextrol Tm

^ Postmarketing Experience
In non-U.S. postmarketing surveillance, the following adverse reactions have been reported during post-approval use of diclofenac sodium topical solution. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Body as a Whole:abdominal pain, accidental injury, allergic reaction, asthenia, back pain, body odor, chest pain, edema, face edema, halitosis, headache, lack of drug effect, neck rigidity, pain
Cardiovascular:palpitation, cardiovascular disorder
Digestive:diarrhea, dry mouth, dyspepsia, gastroenteritis, decreased appetite, mouth ulceration, nausea, rectal hemorrhage, ulcerative stomatitis
Metabolic and Nutritional:creatinine increased
Musculoskeletal:leg cramps, myalgia
Nervous:depression, dizziness, drowsiness, lethargy, paresthesia, paresthesia at application site
Respiratory:asthma, dyspnea, laryngismus, laryngitis, pharyngitis
Skin and Appendages: At the Application Site:contact dermatitis, contact dermatitis with vesicles, dry skin, pruritus, rash; Other Skin and Appendages Adverse Reactions:eczema, rash, pruritus, skin discoloration, urticaria
Special Senses:abnormal vision, blurred vision, cataract, ear pain, eye disorder, eye pain, taste perversion
^ How Supplied/storage And Handling
Diclofenac sodium topical solution USP, 1.5% w/w is supplied as a clear, colorless to slightly pink-orange solution containing 16.05 mg of diclofenac sodium per mL of solution, in a white bottle with a white dropper cap.
^ Geriatric Use
Elderly patients, compared to younger patients, are a greater risk for NSAID-associated serious cardiovascular, gastrointestinal, and/or renal adverse reactions. If the anticipated benefit for the elderly patient outweighs these potential risks, start dosing at the low end of the dosing range, and monitor patients for adverse effects [ see Warnings and Precautions (5.1, 5.2, 5.3, 5.6, 5.14) ].
Of the 911 patients treated with diclofenac sodium in seven controlled, Phase 3 clinical trials, 444 subjects were 65 years of age and over. There was no age-related difference in the incidence of adverse events. Of the 793 patients treated with diclofenac sodium in one open-labeled safety trial, 334 subjects were 65 years of age and over including 107 subjects 75 and over. There was no difference in the incidence of adverse events with long-term exposure to diclofenac sodium for this elderly population.
^ Eye Exposure
Avoid contact of diclofenac sodium with eyes and mucosa. Advise patients that if eye contact occurs, immediately wash out the eye with water or saline and consult a physician if irritation persists for more than an hour.
^ Oral Nonsteroidal Anti-inflammatory Drugs
Concomitant use of oral NSAIDs with diclofenac sodium resulted in a higher rate of rectal hemorrhage, more frequent abnormal creatinine, urea and hemoglobin. Therefore, do not use combination therapy with diclofenac sodium and an oral NSAID unless the benefit outweighs the risk and conduct periodic laboratory evaluations.
^ Laboratory Monitoring
Because serious GI bleeding, hepatotoxicity, and renal injury can occur without warning symptoms or signs, consider monitoring patients on long-term NSAID treatment with a CBC and a chemistry profile periodically [ see Warnings and Precautions (5.2, 5.3, 5.6) ].
^ General Dosing Instructions
Use the lowest effective dosage for the shortest duration consistent with individual patient treatment goals [ see Warning and Precautions (5.2) ].
For the relief of the signs and symptoms of osteoarthritis of the knee(s), the recommended dose is 40 drops per knee, 4 times a day.
Apply diclofenac sodium topical solution to clean, dry skin.
To avoid spillage, dispense diclofenac sodium topical solution 10 drops at a time either directly onto the knee or first into the hand and then onto the knee. Spread diclofenac sodium topical solution evenly around front, back and sides of the knee. Repeat this procedure until 40 drops have been applied and the knee is completely covered with solution.
To treat the other knee, if symptomatic, repeat the procedure.
Application of diclofenac sodium topical solution in an amount exceeding or less than the recommended dose has not been studied and is therefore not recommended.
^ Gastrointestinal Bleeding, Ulceration And Perforation
NSAIDs, including diclofenac, cause serious gastrointestinal (GI) adverse events including inflammation, bleeding, ulceration, and perforation of the esophagus, stomach, small intestine, or large intestine, which can be fatal. These serious adverse events can occur at any time, with or without warning symptoms, in patients treated with NSAIDs. Only one in five patients who develop a serious upper GI adverse event on NSAID therapy is symptomatic. Upper GI ulcers, gross bleeding, or perforation caused by NSAIDs occurred in approximately 1% of patients treated for 3 to 6 months, and in about 2% to 4% of patients treated for one year. However, even short-term NSAID therapy is not without risk.
^Active Ingredient
Capsicum Oleoresin 0.0625% (Containing Capsaicin 0.025%)
^Discontinue Use And Consult A Doctor If
condition worsens or if symptoms persist for more than 7 days or clear up and occur again within a few days. Stop using and get immediate medical attention if experience burning, pain, swelling or blistering of the skin. Rare cases of severe burning or blistering have been reported. If pregnant, breast-feeding or any medical conditions exist, ask a health professional before use.
^Other Information
WARNING: FLAMMABLE PRODUCT
Store in a cool well ventilated area away from heat. Keep away from sparks or open flame.
^ Sun Exposure
Instruct patients to avoid exposure to natural or artificial sunlight on treated knee(s) because studies in animals indicated topical diclofenac treatment resulted in an earlier onset of ultraviolet light-induced skin tumors. The potential effects of diclofenac sodium on skin response to ultraviolet damage in humans are not known.
^Purpose
Topical Analgesic
^Questions Or Comments
Call toll-free 1-877-921-7873 or visit us at www.dermacinrx.com
^6 Adverse Reactions
The following adverse reactions are discussed in greater detail in other sections of the labeling:
^ Studies In Osteoarthritis Of The Knee
The use of diclofenac sodium topical solution for the treatment of the signs and symptoms of osteoarthritis of the knee was evaluated in two double-blind controlled trials conducted in the U.S. and Canada, involving patients treated with diclofenac sodium topical solution at a dose of 40 drops four times a day for 12 weeks. Diclofenac sodium topical solution was compared to topical placebo (2.3% DMSO with other excipients) and/or topical vehicle solution (45.5% w/w DMSO with other excipients), applied directly to the study knee. In both trials, diclofenac sodium topical solution treatment resulted in statistically significant clinical improvement compared to placebo and/or vehicle, in all three primary efficacy variables–pain, physical function (Western Ontario and McMaster Universities LK3.1 OA Index (WOMAC) pain and physical function dimensions) and Patient Overall Health Assessment (POHA)/Patient Global Assessment (PGA). Numerical results are summarized in Tables 4 and 5.
^ Special Precautions
^7 Drug Interactions
See Table 2for clinically significant drug interactions with diclofenac.
^ Hepatotoxicity
In clinical trials, of oral diclofenac-containing products, meaningful elevations (i.e., more than 3 times the ULN) of AST (SGOT) were observed in about 2% of approximately 5,700 patients at some time during diclofenac treatment (ALT was not measured in all studies).
In a large, open-label, controlled trial of 3,700 patients treated with oral diclofenac for 2 to 6 months, patients were monitored first at 8 weeks and 1,200 patients were monitored again at 24 weeks. Meaningful elevations of ALT and/or AST occurred in about 4% of 3,700 patients and included marked elevations (greater than 8 times the ULN) in about 1% of the 3,700 patients. In that open-label study, a higher incidence of borderline (less than 3 times the ULN), moderate (3 to 8 times the ULN), and marked (greater than 8 times the ULN) elevations of ALT or AST was observed in patients receiving diclofenac when compared to other NSAIDs. Elevations in transaminases were seen more frequently in patients with osteoarthritis than in those with rheumatoid arthritis.
Almost all meaningful elevations in transaminases were detected before patients became symptomatic. Abnormal tests occurred during the first 2 months of therapy with diclofenac in 42 of the 51 patients in all trials who developed marked transaminase elevations.
In postmarketing reports, cases of drug-induced hepatotoxicity have been reported in the first month, and in some cases, the first 2 months of therapy, but can occur at any time during treatment with diclofenac. Postmarketing surveillance has reported cases of severe hepatic reactions, including liver necrosis, jaundice, fulminant hepatitis with and without jaundice, and liver failure. Some of these reported cases resulted in fatalities or liver transplantation.
In a European retrospective population-based, case-controlled study, 10 cases of diclofenac associated drug-induced liver injury with current use compared with non-use of diclofenac were associated with a statistically significant 4-fold adjusted odds ratio of liver injury. In this particular study, based on an overall number of 10 cases of liver injury associated with diclofenac, the adjusted odds ratio increased further with female gender, doses of 150 mg or more, and duration of use for more than 90 days.
Physicians should measure transaminases at baseline and periodically in patients receiving long-term therapy with diclofenac, because severe hepatotoxicity may develop without a prodrome of distinguishing symptoms. The optimum times for making the first and subsequent transaminase measurements are not known. Based on clinical trial data and postmarketing experiences, transaminases should be monitored within 4 to 8 weeks after initiating treatment with diclofenac. However, severe hepatic reactions can occur at any time during treatment with diclofenac. If abnormal liver tests persist or worsen, if clinical signs and/or symptoms consistent with liver disease develop, or if systemic manifestations occur (e.g., eosinophilia, rash, abdominal pain, diarrhea, dark urine, etc.), diclofenac sodium should be discontinued immediately.
Inform patients of the warning signs and symptoms of hepatotoxicity (e.g., nausea, fatigue, lethargy, diarrhea, pruritus, jaundice, right upper quadrant tenderness, and "flu-like" symptoms). If clinical signs and symptoms consistent with liver disease develop, or if systemic manifestations occur (e.g., eosinophilia, rash, etc.), discontinue diclofenac sodium immediately, and perform a clinical evaluation of the patient.
To minimize the potential risk for an adverse liver-related event in patients treated with diclofenac sodium, use the lowest effective dose for the shortest duration possible. Exercise caution when prescribing diclofenac sodium with concomitant drugs that are known to be potentially hepatotoxic (e.g., acetaminophen, antibiotics, antiepileptics).
^ Serious Skin Reactions
NSAIDs, including diclofenac, can cause serious skin adverse reactions such as exfoliative dermatitis, Stevens-Johnson Syndrome (SJS), and toxic epidermal necrolysis (TEN), which can be fatal. These serious events may occur without warning. Inform patients about the signs and symptoms of serious skin reactions, and to discontinue the use of diclofenac sodium at the first appearance of skin rash or any other sign of hypersensitivity. Diclofenac sodium is contraindicated in patients with previous serious skin reactions to NSAIDs [ see Contraindications (4) ].
Do not apply diclofenac sodium to open skin wounds, infections, inflammations, or exfoliative dermatitis, as it may affect absorption and tolerability of the drug.
^ Pharmacokinetics
After topical administration to healthy human volunteers of single and multiple maximum doses of diclofenac sodium topical solution, 40 drops (approximately 1.2 mL) to each knee (80 drops total dose), the following diclofenac pharmacokinetic parameters were obtained: (see Table 3 ).
^ Exacerbation Of Asthma Related To Aspirin Sensitivity
A subpopulation of patients with asthma may have aspirin-sensitive asthma which may include chronic rhinosinusitis complicated by nasal polyps; severe, potentially fatal bronchospasm; and/or intolerance to aspirin and other NSAIDs. Because cross-reactivity between aspirin and other NSAIDs has been reported in such aspirin-sensitive patients, diclofenac sodium is contraindicated in patients with this form of aspirin sensitivity [ see Contraindications (4) ]. When diclofenac sodium is used in patients with preexisting asthma (without known aspirin sensitivity), monitor patients for changes in the signs and symptoms of asthma.
^1 Indications And Usage
Diclofenac sodium topical solution, USP is indicated for the treatment of signs and symptoms of osteoarthritis of the knee(s) (1).
^ Cardiovascular Thrombotic Events
Clinical trials of several COX-2 selective and nonselective NSAIDs of up to three years duration have shown an increased risk of serious cardiovascular (CV) thrombotic events, including myocardial infarction (MI) and stroke, which can be fatal. Based on available data, it is unclear that the risk for CV thrombotic events is similar for all NSAIDs. The relative increase in serious CV thrombotic events over baseline conferred by NSAID use appears to be similar in those with and without known CV disease or risk factors for CV disease. However, patients with known CV disease or risk factors had a higher absolute incidence of excess serious CV thrombotic events, due to their increased baseline rate. Some observational studies found that this increased risk of serious CV thrombotic events began as early as the first weeks of treatment. The increase in CV thrombotic risk has been observed most consistently at higher doses.
To minimize the potential risk for an adverse CV event in NSAID-treated patients, use the lowest effective dose for the shortest duration possible. Physicians and patients should remain alert for the development of such events, throughout the entire treatment course, even in the absence of previous CV symptoms. Patients should be informed about the symptoms of serious CV events and the steps to take if they occur.
There is no consistent evidence that concurrent use of aspirin mitigates the increased risk of serious CV thrombotic events associated with NSAID use. The concurrent use of aspirin and an NSAID, such as diclofenac, increases the risk of serious gastrointestinal (GI) events [ see Warnings and Precautions (5.2) ].
^ Mechanism Of Action
Diclofenac has analgesic, anti-inflammatory, and antipyretic properties. The mechanism of action of diclofenac sodium, like that of other NSAIDs, is not completely understood but involves inhibition of cyclooxygenase (COX-1 and COX-2).
Diclofenac is a potent inhibitor of prostaglandin synthesis in vitro. Diclofenac concentrations reached during therapy have produced in vivo effects. Prostaglandins sensitize afferent nerves and potentiate the action of bradykinin in inducing pain in animal models. Prostaglandins are mediators of inflammation. Because diclofenac is an inhibitor of prostaglandin synthesis, its mode of action may be due to a decrease of prostaglandins in peripheral tissues.
^Warnings
For external use only
^When Using This Product
do not get into eyes and avoid contact with other mucous membranes. If contact occurs or if pain, discomfort or skin redness occurs, continually rinse with cool water and seek medical help.
^ Hypertension
NSAIDs, including diclofenac, can lead to new onset of hypertension, or worsening of preexisting hypertension, either of which may contribute to the increased incidence of CV events. Patients taking angiotensin converting enzyme (ACE) inhibitors, thiazide diuretics, or loop diuretics may have impaired response to these therapies when taking NSAIDs [ see Drug Interactions (7) ].
Monitor blood pressure (BP) closely during the initiation of NSAID treatment and throughout the course of therapy.