^ Inhibition Of Bone Growth
The use of tetracycline-class drugs, including minocycline hydrochloride extended-release tablets, during the second and third trimesters of pregnancy, infancy, and childhood up to the age of 8 years may cause reversible inhibition of bone growth. All tetracyclines, including minocycline hydrochloride extended-release tablets, form a stable calcium complex in any bone-forming tissue. A decrease in fibula growth rate has been observed in premature human infants given oral tetracycline in doses of 25 mg/kg every 6 hours. This reaction was shown to be reversible when the drug was discontinued.
Advise the patient of the potential risk to the fetus if minocycline hydrochloride extended-release tablets are used during the second or third trimester of pregnancy [see Use in Specific Populations (8.1, 8.4)].
^ Pharmacodynamics
The pharmacodynamics of minocycline hydrochloride extended-release tablets for the treatment of acne are unknown.
^Package Label-principal Display Panel - Mg ( Tablets Bottle)
NDC 65862-884-30 Rx only Minocycline Hydrochloride Extended-Release Tablets, USP 80 mg* AUROBINDO 30 Tablets
^Package Label-principal Display Panel - Mg ( Tablets Bottle)
NDC 65862-558-30 Rx only Minocycline Hydrochloride Extended-Release Tablets, USP 135 mg* AUROBINDO 30 Tablets
^ Development Of Drug-resistant Bacteria
Bacterial resistance to tetracyclines may develop in patients using minocycline hydrochloride extended-release tablets. Because of the potential for drug-resistant bacteria to develop during the use of minocycline hydrochloride extended-release tablets, it should be used only as indicated.
^ Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling (Patient Information).
Patients taking minocycline hydrochloride extended-release tablets should receive the following information and instructions:
Administration Instructions
Serious Skin/Hypersensitivity Reactions
Tooth Discoloration and Enamel Hypoplasia
Inhibition of Bone Growth
Clostridioides difficile- Associated Diarrhea (Antibiotic-Associated Colitis)
Hepatotoxicity
Central Nervous System Effects
Idiopathic Intracranial Hypertension
Autoimmune Syndromes
Photosensitivity
Tissue Hyperpigmentation
Lactation
Distributed by:
Aurobindo Pharma USA, Inc.
279 Princeton-Hightstown Road
East Windsor, NJ 08520
Manufactured by:
Aurobindo Pharma Limited
Hyderabad-500 032, India
Revised: 04/2025
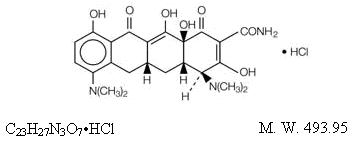
^ Description
Minocycline hydrochloride, a semi synthetic derivative of tetracycline, is [4S-(4α,4aα,5aα,12aα)]-4,7-Bis(dimethylamino)-1,4,4a,5,5a,6,11,12a-octahydro-3,10,12,12a-tetrahydroxy-1,11-dioxo-2-naphthacenecarboxamide monohydrochloride. The structural formula is represented below:
Minocycline hydrochloride extended-release tablets USP for oral administration contain minocycline hydrochloride, USP equivalent to 45 mg, 55 mg, 65 mg, 80 mg, 90 mg, 105 mg, 115 mg, and 135 mg of minocycline. In addition, 45 mg, 55 mg, 65 mg, 80 mg, 90 mg, 105 mg, 115 mg, and 135 mg tablets contain the following inactive ingredients: colloidal silicon dioxide, hypromellose, lactose monohydrate, magnesium stearate, titanium dioxide, and triacetin.
Meets USP dissolution test 2 for 45 mg, 90 mg and 135 mg.
FDA approved dissolution test specifications differ from USP for 55 mg, 65 mg, 80 mg, 105 mg and 115 mg.
^ Tissue Hyperpigmentation
Tetracycline-class antibiotics are known to cause hyperpigmentation. Tetracycline therapy may induce hyperpigmentation in many organs, including nails, bone, skin, eyes, thyroid, visceral tissue, oral cavity (e.g., teeth, mucosa, alveolar bone), sclerae, and heart valves. Skin and oral pigmentation has been reported to occur independently of time or amount of drug administration, whereas other tissue pigmentation has been reported to occur upon prolonged administration. Skin pigmentation includes diffuse pigmentation as well as over sites of scars or injury.
^ Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The following table summarizes selected adverse reactions reported in clinical trials at a rate of ≥1% for minocycline hydrochloride extended-release tablets and higher than placebo.
^ Overdosage
Minocycline is not removed in significant quantities by hemodialysis or peritoneal dialysis. In case of overdosage, discontinue minocycline hydrochloride extended-release tablets, treat symptomatically, and institute supportive measures. Call Poison Control Center at 1-800-222-1222 for the latest recommendations.
^ Anticoagulants
Because tetracyclines have been shown to depress plasma prothrombin activity, patients who are on anticoagulant therapy may require downward adjustment of their anticoagulant dosage.
^ Lactation
Risk Summary
Tetracycline-class antibiotics, including minocycline, are present in breast milk following oral administration. There are no data on the effects of minocycline on milk production. Because of the potential for serious adverse reactions, including tooth discoloration and inhibition of bone growth, advise patients that breastfeeding is not recommended during minocycline hydrochloride extended-release tablets therapy and for 4 days after the final dose [see Warnings and Precautions (5.2, 5.3)].
^3 Dosage Forms And Strengths
^ Central Nervous System Effects
Central nervous system side effects including light-headedness, dizziness, or vertigo have been reported with minocycline therapy. Caution patients who experience these symptoms about driving vehicles or using hazardous machinery while on minocycline hydrochloride extended-release tablets. These symptoms may disappear during therapy and usually rapidly disappear when minocycline hydrochloride extended-release tablets are discontinued.
^ Pediatric Use
The safety and effectiveness of minocycline hydrochloride extended-release tablets have been established in pediatric patients 12 years of age and older for the treatment of inflammatory lesions of non-nodular moderate to severe acne vulgaris [see Clinical Studies (14)]. Tooth discoloration and inhibition of bone growth have been observed in pediatric patients [see Warnings and Precaution (5.2, 5.3)]. Use of tetracycline-class antibiotics below the age of 8 is not recommended due to the potential for tooth discoloration [see Warnings and Precautions (5.2)].
Safety and effectiveness of minocycline hydrochloride extended-release tablets have not been established in pediatric patients younger than 12 years of age.
^ Metabolic Effects
The anti-anabolic action of the tetracyclines, including minocycline hydrochloride extended-release tablets, may cause an increase in blood urea nitrogen (BUN). In patients with significantly impaired renal function, higher serum levels of tetracycline-class drugs may lead to azotemia, hyperphosphatemia, and acidosis. If renal impairment exists, lower the total doses of minocycline hydrochloride extended-release tablets, and if therapy is prolonged, monitor serum levels minocycline hydrochloride extended-release tablets.
^4 Contraindications
Minocycline hydrochloride extended-release tablets are contraindicated in patients with history of a hypersensitivity reaction to any of the tetracyclines [see Warnings and Precautions (5.1)].
^ Postmarketing Experience
The following adverse reactions have been reported with minocycline hydrochloride use in a variety of indications. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Skin and hypersensitivity reactions: anaphylaxis, angioedema, DRESS syndrome, erythema multiforme, Stevens-Johnson syndrome, acute febrile neutrophilic dermatosis (Sweet’s syndrome), fixed drug eruptions, balanitis, anaphylactoid purpura, photosensitivity, pigmentation of skin and mucous membranes.
Autoimmune conditions: polyarthralgia, pericarditis, exacerbation of systemic lupus, pulmonary infiltrates with eosinophilia, lupus-like syndrome.
Central nervous system: idiopathic intracranial hypertension, bulging fontanels in infants, decreased hearing.
Endocrine: brown-black microscopic thyroid discoloration, abnormal thyroid function.
Oncology: thyroid cancer.
Oral: glossitis, dysphagia, tooth discoloration.
Gastrointestinal: enterocolitis, pancreatitis, hepatitis, liver failure.
Renal: acute renal failure.
Hematology: hemolytic anemia, thrombocytopenia, eosinophilia.
^ How Supplied/storage And Handling
How Supplied
Minocycline Hydrochloride Extended-Release Tablets USP, 45 mg are gray colored, round shaped, biconvex, film-coated tablets debossed with ‘I’ on one side and ‘95’ on the other side.
Bottles of 30 NDC 65862-554-30
Bottles of 100 NDC 65862-554-01
Bottles of 1,000 NDC 65862-554-99
Minocycline Hydrochloride Extended-Release Tablets USP, 55 mg are pink colored, round shaped, biconvex, film-coated tablets debossed with ‘K’ on one side and ‘6’ on the other side.
Bottles of 30 NDC 65862-883-30
Bottles of 100 NDC 65862-883-01
Bottles of 500 NDC 65862-883-05
Bottles of 1,000 NDC 65862-883-99
Minocycline Hydrochloride Extended-Release Tablets USP, 65 mg are blue colored, modified capsule shaped, biconvex, film-coated tablets debossed with ‘I’ on one side and ‘26’ on the other side.
Bottles of 30 NDC 65862-555-30
Bottles of 1,000 NDC 65862-555-99
Minocycline Hydrochloride Extended-Release Tablets USP, 80 mg are grey colored, modified capsule shaped, biconvex, film-coated tablets debossed with ‘K’ on one side and ‘7’ on the other side.
Bottles of 30 NDC 65862-884-30
Bottles of 100 NDC 65862-884-01
Bottles of 500 NDC 65862-884-05
Bottles of 1,000 NDC 65862-884-99
Minocycline Hydrochloride Extended-Release Tablets USP, 90 mg are yellow colored, modified capsule shaped, biconvex, film-coated tablets debossed with ‘I’ on one side and ‘27’ on the other side.
Bottles of 30 NDC 65862-556-30
Bottles of 100 NDC 65862-556-01
Bottles of 1,000 NDC 65862-556-99
Minocycline Hydrochloride Extended-Release Tablets USP, 105 mg are purple colored, modified capsule shaped, biconvex, film-coated tablets debossed with ‘K’ on one side and ‘8’ on the other side.
Bottles of 30 NDC 65862-885-30
Bottles of 100 NDC 65862-885-01
Bottles of 500 NDC 65862-885-05
Bottles of 1,000 NDC 65862-885-99
Minocycline Hydrochloride Extended-Release Tablets USP, 115 mg are green colored, capsule shaped, biconvex, film-coated tablets debossed with ‘F81’ on one side and plain on the other side.
Bottles of 30 NDC 65862-557-30
Bottles of 1,000 NDC 65862-557-99
Minocycline Hydrochloride Extended-Release Tablets USP, 135 mg are red colored, modified capsule shaped, biconvex, film-coated tablets debossed with ‘I’ on one side and ‘93’ on the other side.
Bottles of 30 NDC 65862-558-30
Bottles of 100 NDC 65862-558-01
Bottles of 1,000 NDC 65862-558-99
Storage
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Handling
Protect from light, moisture, and excessive heat.
Dispense in tight, light-resistant container with child-resistant closure.
^ Geriatric Use
Clinical studies of minocycline hydrochloride extended-release tablets did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and concomitant disease or other drug therapy.
^ Autoimmune Syndromes
Tetracyclines have been associated with the development of autoimmune syndromes. The long-term use of minocycline in the treatment of acne has been associated with drug-induced lupus-like syndrome, autoimmune hepatitis, and vasculitis. Sporadic cases of serum sickness have presented shortly after minocycline use. Symptoms may be manifested by fever, rash, arthralgia, and malaise. Evaluate symptomatic patients. If symptoms occur, immediately discontinue use of minocycline hydrochloride extended-release tablets.
^ Serious Skin/hypersensitivity Reactions
Cases of anaphylaxis, serious skin reactions (e.g., Stevens-Johnson syndrome), erythema multiforme, and drug rash with eosinophilia and systemic symptoms (DRESS) syndrome have been reported postmarketing with minocycline use in patients with acne. DRESS syndrome consists of cutaneous reaction (such as rash or exfoliative dermatitis), eosinophilia, and one or more of the following visceral complications such as: hepatitis, pneumonitis, nephritis, myocarditis, and pericarditis. Fever and lymphadenopathy may be present. In some cases, death has been reported. If this syndrome is recognized, minocycline hydrochloride extended-release tablets should be discontinued immediately.
Fixed drug eruptions have occurred with minocycline and other tetracyclines. Worsening severity upon subsequent administrations, including generalized bullous fixed drug eruption, has been observed with other tetracyclines [see ADVERSE REACTIONS (6.2)]. If severe skin/hypersensitivity reactions occur, discontinue minocycline hydrochloride extended-release tablets and institute appropriate therapy.
^ Laboratory Monitoring
Perform periodic laboratory evaluations of organ systems, including hematopoietic, renal, and hepatic studies.
^Package Label-principal Display Panel - Mg ( Tablets Bottle)
NDC 65862-557-30 Rx only Minocycline Hydrochloride Extended-Release Tablets, USP 115 mg* AUROBINDO 30 Tablets
^ Carcinogenesis, Mutagenesis, Impairment Of Fertility
In a carcinogenicity study in which minocycline hydrochloride was orally administered to male and female rats once daily for up to 104 weeks at dosages up to 200 mg/kg/day, minocycline hydrochloride was associated in both sexes with follicular cell tumors of the thyroid gland, including increased incidences of adenomas, carcinomas and the combined incidence of adenomas and carcinomas in males, and adenomas and the combined incidence of adenomas and carcinomas in females. In a carcinogenicity study in which minocycline hydrochloride was orally administered to male and female mice once daily for up to 104 weeks at dosages up to 150 mg/kg/day, exposure to minocycline hydrochloride did not result in a significantly increased incidence of neoplasms in either males or females.
Minocycline was not mutagenic in vitro in a bacterial reverse mutation assay (Ames test) or CHO/HGPRT mammalian cell assay in the presence or absence of metabolic activation. Minocycline was not clastogenic in vitro using human peripheral blood lymphocytes or in vivo in a mouse micronucleus test.
Male and female reproductive performance in rats was unaffected by oral doses of minocycline of up to 300 mg/kg/day (40 times the MRHD on an AUC comparison basis). However, oral administration of 100 or 300 mg/kg/day of minocycline to male rats (15 to 40 times the MRHD on an AUC comparison basis) adversely affected spermatogenesis. Effects observed at 300 mg/kg/day included a reduced number of sperm cells per gram of epididymis, an apparent reduction in the percentage of sperm that were motile, and (at 100 and 300 mg/kg/day) increased numbers of morphologically abnormal sperm cells. Morphological abnormalities observed in sperm samples included absent heads, misshapen heads, and abnormal flagella.
^ Idiopathic Intracranial Hypertension
Idiopathic intracranial hypertension has been associated with the use of tetracycline-class drugs, including minocycline hydrochloride extended-release tablets. Clinical manifestations of idiopathic intracranial hypertension include headache, blurred vision, diplopia, and vision loss; papilledema can be found on fundoscopy. Women of childbearing age who are overweight or have a history of idiopathic intracranial hypertension are at a greater risk for developing idiopathic intracranial hypertension. Avoid concomitant use of isotretinoin and minocycline hydrochloride extended-release tablets because isotretinoin, a systemic retinoid, is also known to cause idiopathic intracranial hypertension.
Permanent visual loss may exist, even after the medication is discontinued. If visual disturbance occurs during treatment, prompt ophthalmologic evaluation is warranted. Because intracranial pressure can remain elevated for weeks after drug cessation, monitor patients until they stabilize.
^ Penicillin
Because bacteriostatic drugs may interfere with the bactericidal action of penicillin, avoid giving minocycline hydrochloride extended-release tablets in conjunction with penicillin.
^ Superinfection
Use of minocycline hydrochloride extended-release tablets may result in overgrowth of non-susceptible organisms, including fungi. If superinfection occurs, discontinue minocycline hydrochloride extended-release tablets and institute appropriate therapy.
^Package Label-principal Display Panel - Mg ( Tablets Bottle)
NDC 65862-554-30Rx only Minocycline Hydrochloride Extended-Release Tablets, USP45 mg* AUROBINDO 30 Tablets
^ Photosensitivity
Photosensitivity manifested by an exaggerated sunburn reaction has been observed in some individuals taking tetracyclines, including minocycline. Advise patients to minimize or avoid exposure to natural or artificial sunlight (e.g., tanning beds or UVA/B treatment) while using minocycline hydrochloride extended-release tablets. Instruct patients to use sunscreen products and wear protective apparel (e.g., hat) when exposure to sun cannot be avoided.
^ Antacids And Iron Preparations
Absorption of tetracyclines is impaired by antacids containing aluminum, calcium, or magnesium and iron-containing preparations.
^ Clinical Studies
The safety and efficacy of minocycline hydrochloride extended-release tablets in the treatment of inflammatory lesions of non-nodular moderate to severe acne vulgaris was assessed in two 12-week, multi-center, randomized, double-blind, placebo-controlled trials in adult and pediatric subjects 12 years of age and older (Trial 1 and Trial 2). A total of 924 subjects with non-nodular moderate to severe acne vulgaris received minocycline hydrochloride extended-release tablets or placebo for a total of 12 weeks. The mean age of subjects was 20 years and subjects were from the following racial groups: White (73%), Hispanic (13%), Black (11%), Asian/Pacific Islander (2%), and Other (2%).
The two primary efficacy endpoints were:
1) Mean percent change in inflammatory lesion counts from Baseline to 12 weeks.
2) Percentage of subjects with an Evaluator's Global Severity Assessment (EGSA) of clear or almost clear at 12 weeks.
Efficacy results are presented in Table 3.
Minocycline hydrochloride extended-release tablets did not demonstrate any effect on non-inflammatory lesions (benefit or worsening).
^ Pregnancy
Risk Summary
Tetracycline class drugs, including minocycline hydrochloride extended-release tablets may cause permanent discoloration of deciduous teeth and reversible inhibition of bone growth when administered during the second and third trimesters of pregnancy [see Warnings and Precautions (5.2, 5.3) and Use in Specific Populations (8.4)]. A few postmarketing cases of limb reductions have been reported over decades of use; however, the association is unclear. The limited data from postmarketing reports are not sufficient to inform a drug-associated risk for birth defects or miscarriage.
In animal reproduction studies conducted in pregnant rats and rabbits, fetuses with bent limb bones were observed following oral administration of minocycline during organogenesis at systemic exposures 3 and 2 times, respectively, the exposure associated with the maximum recommended human dose (MRHD) (see Data).
If a patient becomes pregnant while taking this drug, advise the patient of the risk to the fetus and to discontinue treatment.
The background risk of major birth defects and miscarriage for the indicated population is unknown. All pregnancies have a background risk of birth defect, loss, or other adverse outcomes. In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2 to 4% and 15 to 20%, respectively.
Data
Human Data
The use of tetracycline class drugs, including minocycline hydrochloride extended-release tablets, during tooth development (second and third trimesters of pregnancy, infancy, and childhood up to the age of 8 years) may cause permanent discoloration of deciduous teeth (yellow-gray-brown). Permanent discoloration of the teeth is more common during long-term use of the drug but has been observed following repeated short-term courses [see Warnings and Precautions (5.2)].
Animal Data
Results of animal studies indicate that tetracyclines cross the placenta, are found in fetal tissues, and can cause delayed skeletal development in the developing fetus. Evidence of embryotoxicity has been noted in animals treated early in pregnancy [see Warnings and Precautions (5.3)].
Minocycline induced skeletal malformations (bent limb bones) in fetuses when administered to pregnant rats and rabbits during the period of organogenesis at doses of 30 mg/kg/day and 100 mg/kg/day, respectively (3 times the MRHD and 2 times the MRHD on an AUC comparison basis, respectively). Reduced mean fetal body weight was observed in studies in which minocycline was administered to pregnant rats at an oral dose of 10 mg/kg/day (approximately equal to the MRHD on an AUC comparison basis).
Minocycline was assessed for effects on peri- and post-natal development of rats in a study that involved oral administration to pregnant rats during the period of organogenesis through lactation at dosages of 5, 10, or 50 mg/kg/day. In this study, body weight gain was significantly reduced in pregnant females that received 50 mg/kg/day (2.5 times the MRHD on an AUC comparison basis). No effects of treatment on the duration of the gestation period or the number of live pups born per litter were observed. Gross external anomalies observed in offspring of animals that received minocycline included reduced body size, improperly rotated forelimbs, and reduced size of extremities. No effects were observed on the physical development, behavior, learning ability, or reproduction of the offspring of animals that received minocycline.
^6 Adverse Reactions
The following clinically significant adverse reactions are described elsewhere in the labeling:
^Package Label-principal Display Panel - Mg ( Tablets Bottle)
NDC 65862-555-30 Rx only Minocycline Hydrochloride Extended-Release Tablets, USP65 mg* AUROBINDO 30 Tablets
^Package Label-principal Display Panel - Mg ( Tablets Bottle)
NDC 65862-556-30 Rx only Minocycline Hydrochloride Extended-Release Tablets, USP 90 mg* AUROBINDO 30 Tablets
^Package Label-principal Display Panel - Mg ( Tablets Bottle)
NDC 65862-885-30 Rx only Minocycline Hydrochloride Extended-Release Tablets, USP 105 mg* AUROBINDO 30 Tablets
^2 Dosage And Administration
The recommended dosage of minocycline hydrochloride extended-release tablets is approximately 1 mg/kg once daily for 12 weeks. Table 1 provides the recommended minocycline hydrochloride extended-release tablets dosage based upon weight ranges.
Higher dosages have not shown to be of additional benefit in the treatment of inflammatory lesions of acne and may be associated with more acute vestibular adverse reactions.
Swallow tablets whole. Do not chew, crush, or split the extended-release tablets.
Administer minocycline hydrochloride extended-release tablets with or without food [see Clinical Pharmacology (12.3)]. Ingestion of food along with minocycline hydrochloride extended-release tablets may help reduce the risk of esophageal irritation and ulceration.
In patients with renal impairment, decrease the daily dosage by either reducing the recommended individual doses and/or by extending the time intervals between doses [see Warnings and Precautions (5.9)].
^ Pharmacokinetics
Minocycline hydrochloride extended-release tablets are not bioequivalent to non-modified release minocycline products. Based on pharmacokinetic studies in healthy adults, minocycline hydrochloride extended-release tablets produce a delayed Tmax at 3.5 to 4 hours as compared to a non-modified release reference minocycline product (Tmax at 2.25 to 3 hours). At steady-state (Day 6), the mean AUC(0–24) and Cmax were 33.32 mcg×hr/mL and 2.63 mcg/mL for minocycline hydrochloride extended-release tablets and 46.35 mcg×hr/mL and 2.92 mcg/mL for minocycline hydrochloride capsules, respectively. These parameters are based on dose adjusted to 135 mg/day for both products. A single-dose, four-way crossover study demonstrated that minocycline hydrochloride extended-release tablets used in the study (45 mg, 90 mg, 135 mg) exhibited dose-proportional pharmacokinetics. In another single-dose, five-way crossover pharmacokinetic study, minocycline hydrochloride extended-release tablets 55 mg, 80 mg, and 105 mg were shown to be dose-proportional to minocycline hydrochloride extended-release tablets 90 mg and 135 mg. When minocycline hydrochloride extended-release tablets were administered concomitantly with a meal that included dairy products, the extent and timing of absorption of minocycline did not differ from that of administration under fasting conditions. Minocycline is lipid soluble and distributes into the skin and sebum.
Susceptibility Testing
For specific information regarding susceptibility test interpretive criteria and associated test methods and quality control standards recognized by FDA for this drug, please see: https://www.fda.gov/STIC.
^ Tooth Discoloration And Enamel Hypoplasia
The use of tetracycline-class drugs, including minocycline hydrochloride extended-release tablets, during tooth development (second and third trimesters of pregnancy, infancy, and childhood up to the age of 8 years) may cause permanent discoloration of the teeth (yellow-graybrown). Permanent discoloration of the teeth is more common during long-term use of tetracycline-class drugs but has been observed following repeated short-term courses. Enamel hypoplasia has also been reported. Use of minocycline hydrochloride extended-release tablets are not recommended during tooth development.
Advise the patient of the potential risk to the fetus if minocycline hydrochloride extended-release tablets are used during the second or third trimester of pregnancy [see Use in Specific Populations (8.1, 8.4)].
^ Hepatotoxicity
Postmarketing cases of serious liver injury, including irreversible drug-induced hepatitis and fulminant hepatic failure (sometimes fatal), have been reported with minocycline use in the treatment of acne. Discontinue minocycline hydrochloride extended-release tablets if liver injury is suspected.
^Package Label-principal Display Panel - Mg ( Tablets Bottle)
NDC 65862-883-30 Rx only Minocycline Hydrochloride Extended-Release Tablets, USP 55 mg* AUROBINDO 30 Tablets
^ Drug/laboratory Test Interactions
False elevations of urinary catecholamine levels may occur due to interference with the fluorescence test.
^1 Indications And Usage
Minocycline hydrochloride extended-release tablets are indicated to treat inflammatory lesions of non-nodular moderate to severe acne vulgaris in patients 12 years of age and older.
Limitations of Use
^ Clostridioides Difficile Associated Diarrhea (antibiotic Associated Colitis)
Clostridioides difficile-associated diarrhea (CDAD) has been reported with nearly all antibacterial agents, including minocycline, and may range in severity from mild diarrhea to fatal colitis.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin-producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, discontinue minocycline hydrochloride extended-release tablets.
^ Mechanism Of Action
The mechanism of action of minocycline hydrochloride extended-release tablets for the treatment of acne is unknown.